Drug hypersensitivity reactions (DHRs) are the adverse effects of drugs that, when taken at doses generally tolerated by normal subjects, clinically resemble allergy. We aimed to assess the prevalence of self-reported DHRs among Lithuanian children and adults and to identify possible risk factors.
Materials and methodsA cross-sectional survey of a population visiting their general practitioners in Vilnius and Kaunas regions of Lithuania was performed. Thirty-five questions on drug allergy symptoms, in addition, food, pollen allergy and family history were included.
Results3222 (60.0%) children and 2148 (40.0%) adults were included in the study. 7.9% of children and 13.8% of adults reported a DHR for at least one drug (p<0.001). 69.8% of children and 47.3% of adults, who indicated DHRs, had skin symptoms. Rate of anaphylaxis was similar in both groups (about 10%). 4.5% of children and 7.3% of adults had DHRs induced by antibiotics and this was the most implicated group of drugs. Significant self-reported risk factors for DHRs were family history of DHRs (OR=6.007, 95%CI 4.756–7.587), pollen allergy (OR=2.0, 95%CI 1.573–2.544), food allergy (OR=1.92, 95%CI 1.505–2.448), female gender (OR=1.439, 95%CI 1.187–1.744) and age (OR=1.017 in favour of adults, 95%CI 1.013–1.021).
ConclusionsThe prevalence of self-reported DHRs in Lithuania is higher among adults than children. Drug-induced skin reactions were the predominant symptom in both groups. Besides female gender and age, a positive family history of DHR and presence of pollen or food allergy may be associated with DHR.
Drug hypersensitivity reactions (DHRs) are the adverse effects of drugs that, when taken at doses generally tolerated by normal subjects, clinically resemble allergy.1 They are almost a daily worry for the clinician. These reactions should only be classified as a drug allergy when a definite immunological mechanism is demonstrated.2
Generally adverse drug reactions are classified into six types: dose-related (Augmented), non-dose-related (Bizarre), dose-related and time-related (Chronic), time-related (Delayed), withdrawal (End of use), and failure of therapy (Failure).3 The majority of adverse drug reactions are type A reactions and they are predictable. Type B reactions are unpredictable and constitute up to one third of all adverse drug reactions according to some epidemiological findings. DHRs belong to type B adverse drug reactions and they can be potentially life threatening.4 The treatment of allergic patients is difficult and more expensive in comparison to non-allergic individuals.5
The exact incidence of drug allergy is not known. In some previous studies adverse drug reactions accounted for 3–6% of all hospital admissions.6 The majority of currently available studies are focused only on selected population groups, and may not represent the situation in general population.
There is also no data on the prevalence of DHRs in Lithuania, so the aim of our study was to assess the incidence of self-reported DHRs in Lithuanian children and adults, and to identify possible risk factors.
Materials and methodsStudy materialA cross-sectional survey of the general population in the Vilnius and Kaunas regions of Lithuania was performed by four general practitioners (one from Kaunas and three from Vilnius), and 12 medical residents from the Vilnius University Hospital Santaros klinikos and Hospital of Lithuanian University of Health Sciences Kauno klinikos. The participants were consecutive patients – adults and children from general practitioner offices during a seven-year period. 5370 subjects who gave an informed consent were included in the study. The study protocol was approved by the local Ethics Committee. A specifically developed self-applied questionnaire was used. It consisted of 35 questions concerning drug-induced allergy symptoms, personal history of food and pollen allergy, and family history of any known allergic reactions. The prevalence of DHRs was calculated by the answer to the question: “Have you ever suffered from any drug-induced allergic reaction?”. Then, a list of various hypersensitivity reactions was proposed to know the type of symptoms: cutaneous (redness, rashes, itch, oedema), nasal (sneeze, stuffy nose), ocular (itch, redness, watery), respiratory (dyspnoea, wheeze, cough), gastrointestinal (nausea, vomit, diarrhoea, stomach ache), cardiovascular (decrease of blood pressure, tachycardia, pallor, sweating, loss of consciousness) and others. Symptoms involving at least two organ systems were defined as anaphylaxis. Reactions occurring within 1h after administering the drug were considered as immediate-type DHRs, whereas delayed reactions were considered as non-immediate-type DHRs.7 The questionnaire included questions about the brand name and class of the drug involved in the reaction. All the fulfilled questionnaires were analysed by one medical statistician at the Centre of Allergology and Pulmonology, Vilnius University Hospital Santaros klinikos.
Statistical analysisThe data was analysed using SPSS software (version 17.0). Categorical variables were expressed as frequencies and percentages; age was expressed as the median and interquartile range (Q1–Q3). Categorical variables were compared using the chi-square test. The loess method for plotting trends was used in detecting the prevalence of different types of allergy. Forward stepwise logistic regression was used to identify the risk factors for DHRs. Odds ratio (OR) with 95% confidence intervals (CIs) were calculated for self-reported risk factor analysis. A value of p<0.05 was considered statistically significant. All reported p-values were two-tailed.
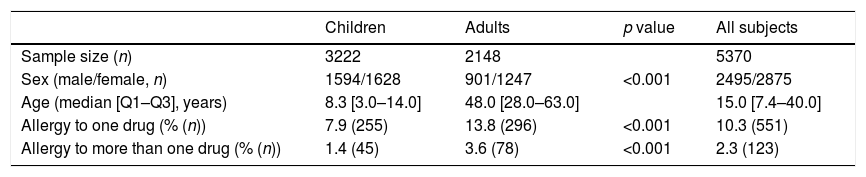
ResultsStudy characteristics647 subjects from 5370 participants who fulfilled questionnaires have reported clinical signs similar to allergy: 551 respondents (10.3% of studied subjects) reported to be allergic to one drug, and 123 (2.3%) subjects declared about multidrug allergy. The children or their parents filled out 3222 (60%) of the questionnaires, out of which 1628 (50.53%) were girls with a median age of 8.3 [3.0–14.0] years. 2148 of all respondents were adults: 1247 (58.05%) women with a median age of 48.0 [28.0–63.0] years. In the children group, the prevalence of self-reported DHRs to one drug was significantly lower than in adults (Table 1). DHRs to more than one drug were reported more frequently in adults than in children (Table 1).
Characteristics of the study population.
| Children | Adults | p value | All subjects | |
|---|---|---|---|---|
| Sample size (n) | 3222 | 2148 | 5370 | |
| Sex (male/female, n) | 1594/1628 | 901/1247 | <0.001 | 2495/2875 |
| Age (median [Q1–Q3], years) | 8.3 [3.0–14.0] | 48.0 [28.0–63.0] | 15.0 [7.4–40.0] | |
| Allergy to one drug (% (n)) | 7.9 (255) | 13.8 (296) | <0.001 | 10.3 (551) |
| Allergy to more than one drug (% (n)) | 1.4 (45) | 3.6 (78) | <0.001 | 2.3 (123) |
Self-reported DHRs were more prevalent among women as compared to men: DHR to at least one drug was reported by 17.5% of women in comparison to 8.7% of men (p<0.001), and multi-DHRs 4.9% and 1.9%, respectively (p<0.001). There was no statistically significant difference in gender among children, neither in allergy to at least one drug (8.4% and 7.4%), nor for allergy to more than one drug (1.4% and 1.4%).
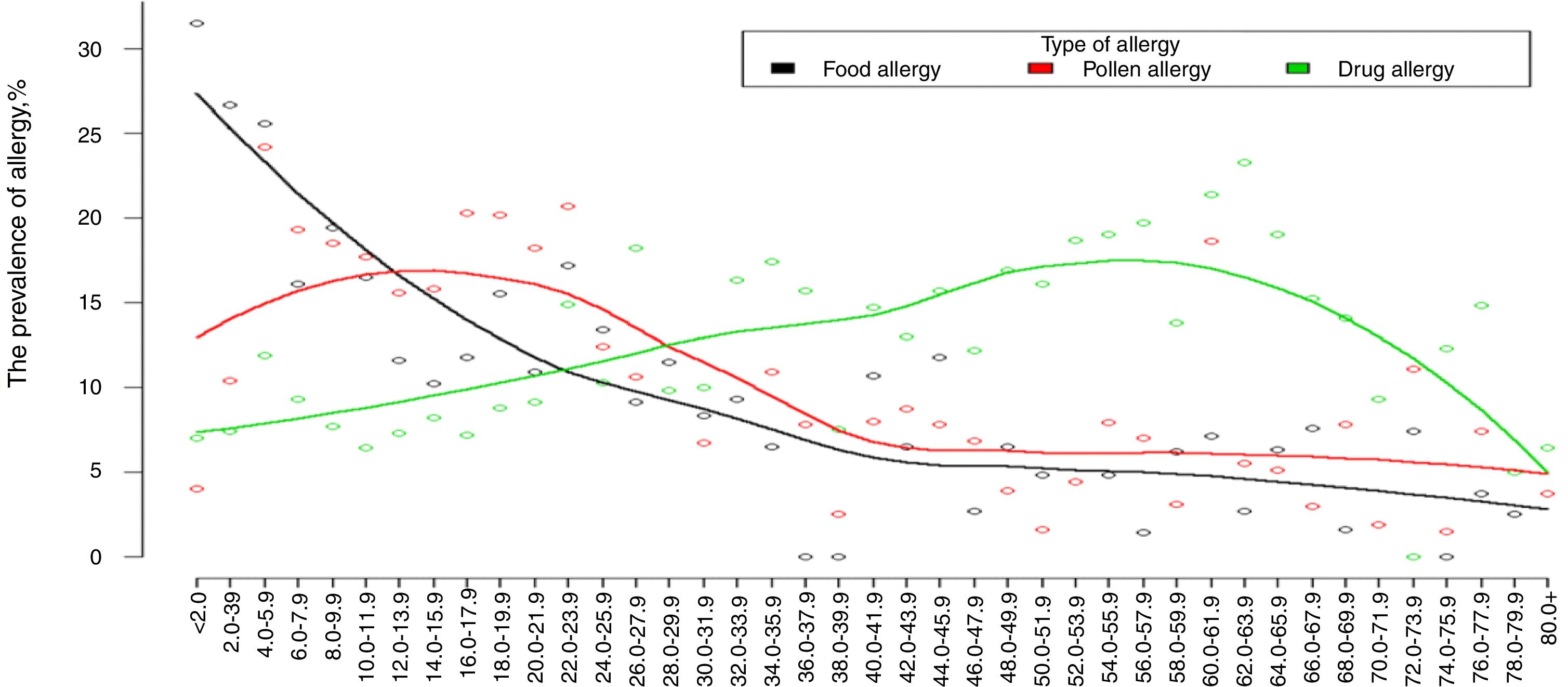
The prevalence of self-reported drug allergy is increasing with the age starting at 20 years and reaching its maximum at 45 and 65 years (Fig. 1).
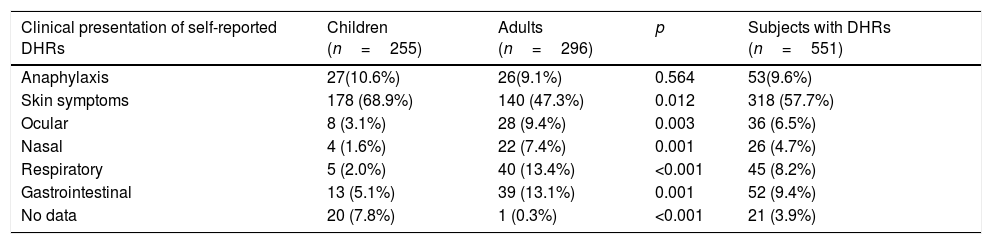
Type of clinical manifestation of self-reported drug allergySkin reactions were reported as the most frequent clinical manifestation of DHRs. In the study, 551 subjects with self-reported DHRs (178 from 255 (68.9%) children and 140 from 296 (47.3%) adults) had skin symptoms such as redness, rashes, itch, or oedema. In both groups these symptoms were reported more frequently than other clinical signs (p=0.012). Other clinical symptoms were more frequent in adults than in children: ocular complaints numbered 28 (9.4%) adults and eight (3.1%) children (p=0.003), nasal symptoms – 22 (7.4%) adults and four (1.6%) children (p=0.001), respiratory symptoms – 40 (13.4%) adults and five (2.0%) children (p<0.001), gastrointestinal symptoms – 39 (13.1%) adults and 13 (5.1%) children (p=0.001). The rate of self-reported drug-induced anaphylaxis was similar in adults and children (9.1% vs. 10.6%).
Assessment of the time between drug intake and manifestation of drug-induced reaction showed that immediate-type reaction was more common in adults than in children (178 (60.1%) vs. 66 (25.7%), p<0.001). It is noteworthy that 14.9% of the respondents did not know the exact time of the reaction onset, so they were not included in this analysis (Table 2).
Type of drug-induced clinical manifestation in Lithuanian children and adults by self-reported survey.
| Clinical presentation of self-reported DHRs | Children (n=255) | Adults (n=296) | p | Subjects with DHRs (n=551) |
|---|---|---|---|---|
| Anaphylaxis | 27(10.6%) | 26(9.1%) | 0.564 | 53(9.6%) |
| Skin symptoms | 178 (68.9%) | 140 (47.3%) | 0.012 | 318 (57.7%) |
| Ocular | 8 (3.1%) | 28 (9.4%) | 0.003 | 36 (6.5%) |
| Nasal | 4 (1.6%) | 22 (7.4%) | 0.001 | 26 (4.7%) |
| Respiratory | 5 (2.0%) | 40 (13.4%) | <0.001 | 45 (8.2%) |
| Gastrointestinal | 13 (5.1%) | 39 (13.1%) | 0.001 | 52 (9.4%) |
| No data | 20 (7.8%) | 1 (0.3%) | <0.001 | 21 (3.9%) |
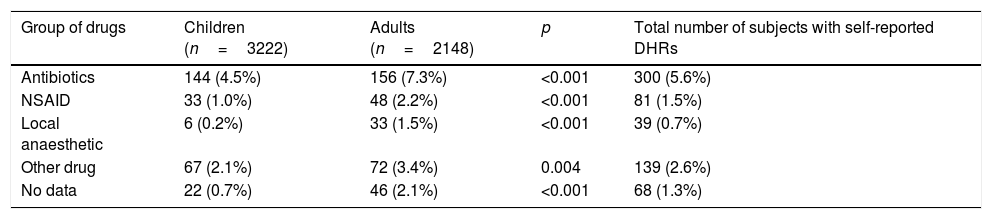
The group of drugs most frequently implicated in the self-reported DHRs was antibiotics. 144 (4.5%) children and 156 (7.3%) adults had DHRs attributed to this class of drugs (p<0.001, compared to the drugs with different chemical composition). Non-steroid anti-inflammatory drugs (NSAIDs) were indicated as the second frequent cause of self-reported DHRs, particularly among adults – 48 (2.2%) cases. The third most often cause of reported DHRs was related to local anaesthetics, with a higher incidence rate among adults than children (33 (1.5%) and six (0.2%), respectively) (Table 3).
Culprit drugs and prevalence of self-reported DHRs in Lithuanian children and adults.
| Group of drugs | Children (n=3222) | Adults (n=2148) | p | Total number of subjects with self-reported DHRs |
|---|---|---|---|---|
| Antibiotics | 144 (4.5%) | 156 (7.3%) | <0.001 | 300 (5.6%) |
| NSAID | 33 (1.0%) | 48 (2.2%) | <0.001 | 81 (1.5%) |
| Local anaesthetic | 6 (0.2%) | 33 (1.5%) | <0.001 | 39 (0.7%) |
| Other drug | 67 (2.1%) | 72 (3.4%) | 0.004 | 139 (2.6%) |
| No data | 22 (0.7%) | 46 (2.1%) | <0.001 | 68 (1.3%) |
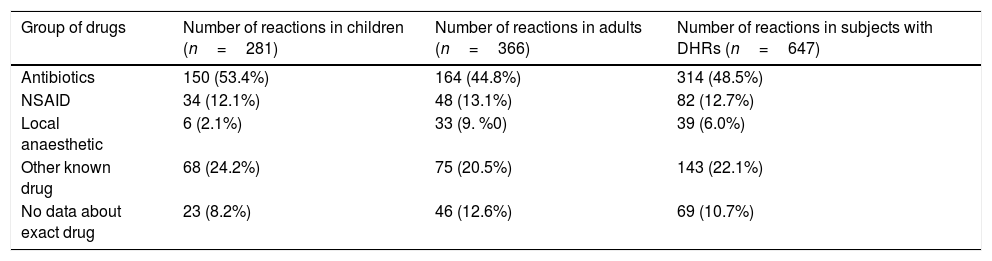
In our study, 551 subjects declared a total number of 647 clinical cases (281 in children and 366 in adults) related to adverse drug reaction, which might be allergy. Although skin rush or other type of adverse reaction to an antibiotic was observed in the past, especially in children, the same or similar group of drugs has been prescribed for the treatment without additional allergological examination. Self-reported antibiotic allergy in adults was more frequent than in children, yet the total number of reported clinical cases was slightly higher in children (164 (44.8%) and 150 (53.4%), respectively) (Table 4).
Incidence of clinical cases of DHRs according to the group of drugs.
| Group of drugs | Number of reactions in children (n=281) | Number of reactions in adults (n=366) | Number of reactions in subjects with DHRs (n=647) |
|---|---|---|---|
| Antibiotics | 150 (53.4%) | 164 (44.8%) | 314 (48.5%) |
| NSAID | 34 (12.1%) | 48 (13.1%) | 82 (12.7%) |
| Local anaesthetic | 6 (2.1%) | 33 (9. %0) | 39 (6.0%) |
| Other known drug | 68 (24.2%) | 75 (20.5%) | 143 (22.1%) |
| No data about exact drug | 23 (8.2%) | 46 (12.6%) | 69 (10.7%) |
In the group of self-reported allergy to antibiotics, 220 cases out of 314 (70.1%) were related to beta-lactams. Among the studied antibiotics, beta-lactams were responsible for clinical reactions in 107 (71.3%) children and in 113 (68.9%) adults.
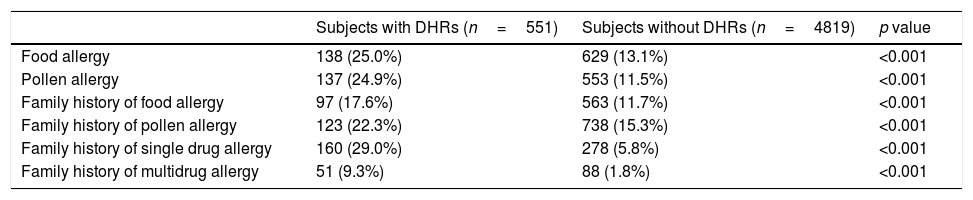
Self-reported risk factor analysisFor the evaluation of possible self-reported risk factors of drug allergy two groups were compared: subjects with DHRs and without DHRs. Food and pollen allergy, family history of food and pollen allergy, family history of drug allergy were reported more frequently in subjects with self-reported DHRs than subjects without DHRs (Table 5). The individuals with mono or multidrug hypersensitivity reported drug allergy in their families more often than subjects without DHRs. Forward stepwise logistic regression analysis showed that family history of DHRs (OR=6.007, 95%CI 4.756–7.587), personal pollen allergy (OR=2.0, 95%CI 1.573–2.544), personal food allergy (OR=1.92, 95%CI 1.505–2.448), female gender (OR=1.439, 95%CI 1.187–1.744) and age (OR=1.017 in favour of adults, 95%CI 1.013–1.021) are significant self-reported risk factors for self-reported DHRs.
Prevalence of different self-reported allergies and family history of allergy in the groups with DHRs and without DHRs.
| Subjects with DHRs (n=551) | Subjects without DHRs (n=4819) | p value | |
|---|---|---|---|
| Food allergy | 138 (25.0%) | 629 (13.1%) | <0.001 |
| Pollen allergy | 137 (24.9%) | 553 (11.5%) | <0.001 |
| Family history of food allergy | 97 (17.6%) | 563 (11.7%) | <0.001 |
| Family history of pollen allergy | 123 (22.3%) | 738 (15.3%) | <0.001 |
| Family history of single drug allergy | 160 (29.0%) | 278 (5.8%) | <0.001 |
| Family history of multidrug allergy | 51 (9.3%) | 88 (1.8%) | <0.001 |
DHRs: drug hypersensitivity reactions.
We assessed the prevalence of self-reported drug allergy in Lithuania and compared these data between adults and children. In our study, 13.8% of adults and 7.9% of children answered “yes” to the question about their drug-induced allergy-like reactions. Data about self-reported DHRs have rarely been reported in the literature. We did not succeed in finding any previously performed study similar to ours, which is why our discussion is mostly based on the results available from separate studies in children and in adults, and from the studies performed in a general population.
According to the scientific literature, prevalence of drug hypersensitivity in adults and children varies between different studies. Data about DHRs and antibiotic usage in the United States was extracted from the electronic health records of 411543 patients of different age; and 165446 drug allergy cases were revealed.7 13.9% of the analysed population reported “one drug allergy”.7 A study performed in Portugal adults showed the lower prevalence of self-reported drug allergy (7.8%) compared to our results.8 According to another cross-sectional study, which was performed in adults from Turkey, the prevalence of self-reported immediate type DHRs was 11.8%,9 close to the data from adults of our study. One study performed in Mexican young adults showed that the prevalence of self-reported drug allergy was 12%,10 which is similar to the results of our study. The prevalence of DHRs in medical students from Turkey was 4.7%.11 A comparison of our results with data from a previously published study in Lithuanian health care workers showed that drug allergy symptoms were more common in health care workers than in the general population.12 The prevalence of self-reported drug allergy in a medical setting was 16.18%, and it is higher than the prevalence in adults (13.8%) from the present study.
Based on the study, which was carried out in Portuguese children, the prevalence of self-reported DHRs was 10.2%.13 In a study performed in Germany, a self-reported questionnaire was distributed to all parents who admitted to hospital with their children during a six-month period.14 The life-time prevalence of drug reactions was identified at 7.5% of respondents, six of the reported reactions were classified as serious and three reactions were anaphylactic (0.2%).14 It is interesting to note that self-reported anaphylaxis in Lithuanian children was more prevalent than in the study from Germany as the results of our study show 10.6%. A similar cross-sectional study, like our survey, was performed in Turkey. The prevalence of parental-reported drug allergy in children was 2.8%,15 which is lower than in Lithuania. In the study of Singaporean children aged 7–16 years, the prevalence of DHRs was also lower than in our study – 5.4%.16 An exploratory study of internationally collected individual case safety reports (in total of 3472183 reports) did analysis of adverse drug reactions,17 and revealed that 7.7% of reports were for these reactions in children (0–17 years).
Several studies in the literature have investigated the possible risk factors for drug allergy. In agreement with our study, the most common risk factor was female sex.7–9,11,17,18 We also found that age is a self-reported risk factor for drug allergy. Some other authors provided almost similar data.7,8,11 However, some other studies did not present a significant difference in the prevalence of DHRs between different age and gender groups.14,15 In agreement with our findings, previous studies performed in Turkey and Mexico revealed that a history of drug hypersensitivity in subjects’ family was associated with an increased self-reporting of drug allergy.10,11 Other risk factors for drug hypersensitivity reported in the scientific literature are asthma, allergic rhinitis, eczema, heart disease, hypertension, diabetes mellitus and overuse of drugs.9,11 We found that the presence of pollen or food allergy are self-reported risk factors for DHRs. According to the report from the paediatric task force of the EAACI Drug Allergy Interest Group, infection can be a risk factor of DHRs as well as other factors can be important for the differential diagnosis of DHRs19; however, we did not aim to analyse these factors in our study.
In our study, we found that the most common symptom of self-reported drug allergy was skin impairment. Numerous studies with adults also showed that the most prevalent clinical symptom related to DHRs was cutaneous reaction.8,9,11 In our study, skin symptoms were indicated most commonly as a drug-induced reaction in both groups, but more prevalent in children (68.9% and 47.3%, respectively). According to the exploratory study using VigiBase, skin and subcutaneous tissue disorders were predominant in adults and children, but also were more prevalent in children.17 Other studies with children also revealed that cutaneous reactions were the most frequent symptom of DHRs.13–15 Interestingly, the rate of gastrointestinal symptoms of DHRs was 21% in Turkey15; whereas in Lithuanian children, gastrointestinal reactions were reported only by 5.1% of respondents.
The most implicated group of drugs in our study was antibiotics (5.6%), particularly beta-lactams, and NSAIDs (1.5%). Studies performed in adults from Portugal and Turkey showed very similar results.8,15 In many studies it was found that antibiotics are the main group of drugs, which may cause hypersensitivity.7,11,14,16,17 According to Rebelo Gomes et al., beta-lactams caused the largest number of reactions in children13; this finding corresponds to our results.
It is known that drug hypersensitivity can be confirmed only after careful evaluation by a specialist in allergy.10 In a Portuguese study after drug provocation test, only a few of these reactions were attributed to DHRs.13 The majority of the subjects (94%) who indicated having DHRs could actually tolerate the initially suspected drug.14 A previous Lithuanian study showed that only 17% of adults with suspected drug hypersensitivity were referred to an allergist for the evaluation of a true diagnosis.20 A large number of adverse reactions induced by drugs is frequently reported as allergy, especially in the paediatric population, and need to be carefully evaluated to establish the true diagnosis.20
In summary, the prevalence of self-reported DHRs in Lithuania is higher among adults than children. Drug-induced skin reactions were the predominant symptom in both groups. 10% of children and adults described self-reported drug-induced reaction as anaphylaxis. Besides female gender and age, a positive family history of DHRs and the presence of pollen or food allergy seem to be important self-reported risk factors for DHRs. However, a thorough drug allergy workup is needed since many of these self-reported reactions may be false DHRs after all.
Conflict of interestThe authors have no conflict of interest to declare.
The authors would like to express their gratitude to Malyte J, Drusyte S, Silkina O, Girutyte J, Kavaliauskaite L, Kunigelyte A, Butiene I and Cerniauskas K from Vilnius University and Vilnius University Hospital Santaros klinikos, and Budryte B, Ragaisiene S, Gasiuniene E and Bajoriuniene I from the Hospital of Lithuanian University of Health Sciences Kauno klinikos for the collection of questionnaires, and Puronaite R from Vilnius University Hospital Santaros Klinikos for statistical analysis.