INTRODUCTION
In previous papers it was demonstrated that a bat feces extract (BAT) was capable to elicit a specific IgG response in rabbits, a specific IgE in atopics suffering perennial rhinitis and asthma and a hypersensitivity pneumonitis in an animal experimental model1,2,3.
Peptidases are classified in serine, cysteine, aspartyl and metalloproteinases, according to 1): the reaction that they catalyze;2: the chemical nature of the catalytic site and3 the evolutionary relationship revealed by their structures4,5.
In this paper, we present evidence indicating that BAT contain serine-like proteinases with gelatinolytic properties which might be involved in their immunogenicity.
MATERIAL AND METHODS
Antigen
BAT was collected as previously described 1,2 and the extract was prepared with these modifications. Three grams of the feces were defatted with sulphuric ether and after its total evaporation at room temperature the BAT was treated with 100 ml of buffer containing 70 mM NaCl, 20 mM CO3HNa at pH 8,5 with gentle shaking during 48 hs at 4° C6.
The extract was centrifuged and the supernatant was dialysed against distilled water 3 times during 24 hs. Then 5 % glycerol was added and the extract was sterilized with Millipore filters and stored at 20° C. The Bradford method was applied to establish the protein content of the BAT7.
Enzymatic activity assay
Minigels of 10 x 10 cm each and 1,5 mm thick composed of 12 % acrylamide were made as described by Laemmli8 with gelatin at a final concentration of 0,2 %. They were run at 130 V for 2 hs. When the bromophenol blue used as a marker reached the bottom, the run was stopped and the gels were washed twice in distilled water with Triton-X-100, 0,15 % for 15 min each, then incubated at 37° C in 0,1 % 2-[N-morpholino] ethane sulfonic acid (MES) buffer at pH 6, in Tris AcH 100 mM pH 3,5 and Tris ClH 100 mM pH 8,5 containing 0,5 mM dithiothreitol (DTT).
The reaction was stopped and the remaining protein was stained by incubation at room temperature with 0,25 Coomasie brilliant blue R-250 in methanol-acetic acid-water 5:1:5 (v/v/v).
After destaining in methanol 20 % and acetic acid 10 %, the active bands were observed as colorless over a blue background.
Inhibitory assays
The washing and incubation of the gels were done with and without the protease inhibitors.
The solutions employed were E 64 (L-trans-epoxysuccinylleucylamido [4-guanidino]-butane) 20 μM; tosyl-lysyl-chloro-methyl-ketone (TLCK) 100 μM; tosyl-phenyl-alanyl-chloro-methyl-ketone (TPCK) 1 mM; phenyl-methyl-sulphonyl-fluoride (PMSF) 10 mM; leupeptin 100 μM; o-phenantroline 1 mM and pepstatin-A 2 μM9,10.
The molecular weight markers were phosphorylase-b (97,4 kDa), bovine serum albumin (BSA) (66,2 kDa), ovoalbumin (45 kDa),carbonic anhydrase (31 kDa), trypsin inhibitor (21,5 kDa) and lysozyme (14,4 kDa). For activity gels, the samples were not reduced nor boiled before loading.
Western blots
The samples with or without β-mercaptoethanol and boiling were run in 15 % standard polyacrylamide gel in the presence of SDS (SDS-PAGE), electrotransfered to nitrocellulose sheets, blocked for 2 h with a solution containing 2 % fatty acid-free BSA, 0,01 % v/v Tween-20, PBS pH 7,2 and then incubated overnight with rabbit polyclonal anti-BAT serum 1/400 and human sera 1/20. After overnight incubation with the rabbit or human antisera, respectively, and repeated washing the sheets were treated with 1/3000 goat anti-rabbit IgG horseradish peroxidase conjugate or 1/500 rabbit anti-human IgE specific for ε-chains peroxidase conjugate at room temperature during 2 hs.. The chromagenic detection was developed using α-chloronaphtol and hydrogen peroxide11.
RESULTS
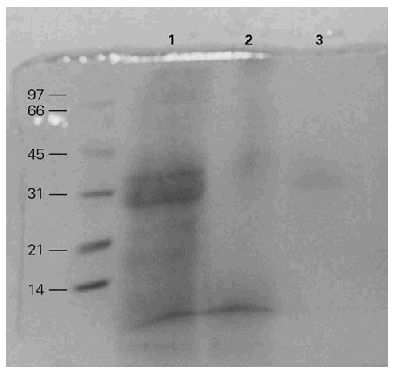
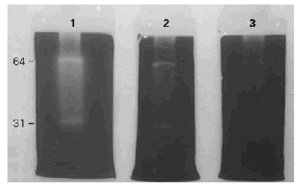
BAT in SDS-PAGE showed 6 to 8 bands between 21 to 97 kDa (fig. 1).
Figure 1.--SDS-PAGE of the BAT extract in different chemical conditions. MW: molecular weight markers. 1: BAT run in reduction conditions. 2: BAT run without reduction conditions. 3: Dilution of BAT run in reduction conditions. Different bands are detected between 21 and 97 kDa.
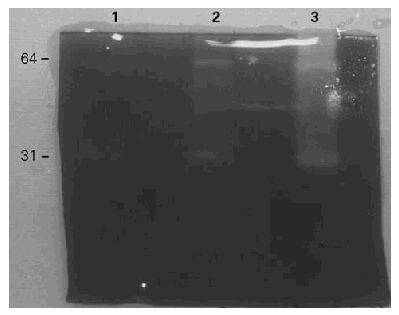
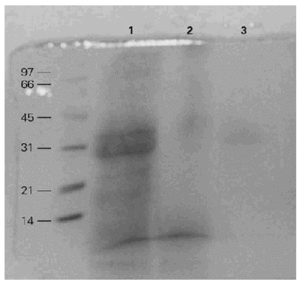
The gelatinolytic activity of the BAT in SDS-PAGE with co-polymerized gelatin as substrate was recorded. The proteolytic activity pattern of BAT was preliminarily analyzed at three different pH levels, 3,5, 6 and 8,5. The highest enzyme activity was at pH 8,5 with less activity at pH 6 and almost no activity at pH 3,5 (fig. 2).
Figure 2.--Gelatinolytic activity of the BAT extract at different pHs. 1: pH: 3,5. 2: pH: 6. 3: pH: 8,5. The highest activity is seen at pH 8,5.
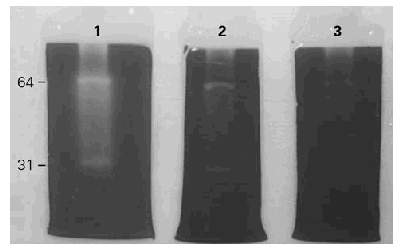
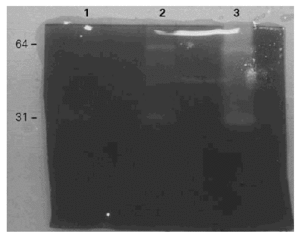
Total activity pattern at pH 8,5 was highly sensitive to TLCK and PMSF while the major and broad band (65 kDa) and the minor (31 kDa) showed the same inhibition pattern. Hence, we tentatively characterized this enzyme as a trypsin-like serine protease (fig. 3).
Figure 3.--Inhibition of the gelatinolytic activity. 1: control without inhibitors. 2: inhibited by PMSF. 3: inhibited by TLCK. Total inhibition is obtained with TLCK.
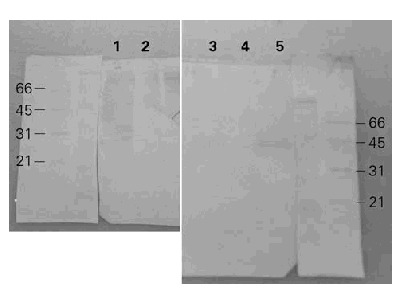
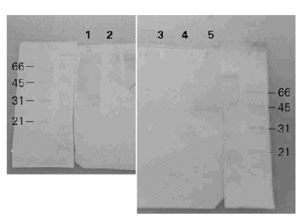
When the BAT was separated by SDS-PAGE, transferred to nitrocellulose and incubated with a polyclonal rabbit anti-BAT serum, the 6 to 8 bands of apparent molecular weights between 21 to 97 kDa showed immunoreactivity (fig. 4). The same results were obtained with or without DTT as a reductor agent in the sample suggesting that disulfide bonds must be absent in these immunogenic bands. On the other hand, when a human anti-BAT serum was employed only the bands of 21 and 40 kDa with gelatinolytic protease activity seemed to be associated with immunoreactivity although many different proteins may be present in each broad band.
Figure 4.--Western-blots with rabbit and human sera. Lanes 1 and 2: incubation with polyclonal rabbit anti-BAT and anti-IgG as a secondary antibody. Lanes 3, 4 and 5: incubation with polyclonal human anti-BAT and anti-IgE as a secondary antibody. With the rabbit anti-BAT serum several bands are seen between 29 and 97 kDa meanwhile with the human anti-BAT serum only two bands are recognized at 21 and 40 kDa.
DISCUSSION
Several major allergens in the extracts of insects possess protein hydrolase properties. Also, acid phosphatase activities correlate well with allergenic potency in pollen extracts. Previous studies showed the identification of a novel serin-protease with allergenic activity from Dermatophagoides pteronyssinus12.
Elsewhere, we described the correlation between some proteases with gelatinolytic activity and the allergenicity of house-dust mite and cockroach extracts13.
We suppose that this is the first report about the trypsin-like serine protease with gelatinolytic activity of a extract of BAT feces of mammalian origin and not from an avian source14. Meanwhile all the proteins separated by SDS-PAGE showed immunoreactivity in the Western blots with a polyclonal rabbit anti BAT serum only 2 bands (21 and 40 kDa) reacted with the human atopic sera suggesting a correlation between allergenicity and gelatinolytic protease activity.
The presence of serine-like proteases with gelatinolytic activity in the bat feces is intriguing and led us to suspect the existence of enzymatic products coming from the digestive tract of the bat, or exudated serum proteins or metabolic products deriving from the daily ingestion of the bat. It was also taking into account the relevant importance that tropomiosin had gained in the last decade as a common molecule present in several arthropods, molluscs and mammalians although the physicochemical properties are different.
It is very important to define the proteases present in all these extracts and to determine their ability to degrade other proteins of the same extract or in mixtures of allergen extracts.
Their role in the immunopathological aspects of rhinitis and asthma requires further investigation.