INTRODUCTION
Specific Immunotherapy (SIT) is widely accepted as a causal treatment of type I allergy. In particular, short-term SIT offers a convenient option and supports compliance in children and adolescents. A recent advance in SIT has been provided by the incorporation of an innovative adjuvant, 3-deacylated monophosphoryl lipid A (MPL® adjuvant, Corixa) in the formulation of allergy vaccines. Clinically successful SIT is now thought to result from either a redressed Th1/Th2 cell balance by promoting a Th1 response which produces cytokines that depress Th2 activity (probably mediated by IL-12), or by a down-regulation induced by IL-10 from regulatory T-cells, ie immunological tolerance1. MPL® adjuvant is a purified, detoxified glycolipid derived from the cell walls of Salmonella minnesota2 and has been shown to induce Th1-like immunological profiles in both pre-clinical3 and clinical4 studies.
Clinical trials of specific immunotherapy with formulations containing MPL® adjuvant have so far proved encouraging. A double-blind, placebo-controlled (DBPC) multi-centre phase III study of SIT with MPL® adjuvant incorporated in a vaccine containing grass and rye pollen allergoids adsorbed to L-tyrosine (Pollinex® Quattro, Bencard Allergie/Allergy Therapeutics Ltd) showed good efficacy after a course of only four injections5; a further DBPC study using tree pollen allergoids showed similar success6. Efficacy in these studies was clearly indicated by symptom and medication scoring, underlined by additional efficacy parameters. Following therapy, skin test sensitivity was found to decrease and specific IgE antibody levels only increased in the placebo groups during the pollen season. Tolerability was also good in both studies. However, these data carried the limitation of a therapy restriction to adult patients only. There are particular reasons to apply SIT for allergic disease in children, because the treatment is thought to be more effective than in adults7, and there is evidence to suggest that it may impede progression from allergic rhinoconjunctivitis to asthma8. The composition of these vaccines also lends confidence in providing safety and efficacy. The use of allergoids reduce allergen-specific IgE reactivity whilst maintaining induction of specific IgG9,10. Also, recent reviews have indicated encouraging safety profiles of the depot adjuvant L-tyrosine11 and MPL® adjuvant12.
Therefore in a further examination of treatment outcomes, an open multi-centre study was employed to evaluate these vaccines for efficacy and tolerability in children and adolescents with allergic sensitivities to tree or grass pollens. Efficacy measurements comprised: symptom and medication scoring (global assessment), skin prick test reactivity and IgG/IgE antibody responses. Tolerability was monitored by incidence of adverse events and safety parameters.
METHODS
Patients were divided into two groups according to their allergic sensitivities. Patients sensitive to grass pollen (n = 26) were treated in five allergy clinics in Portugal. Diagnosis and inclusion criteria were: male or female patients aged between 6-17 with seasonal symptoms due to grass pollen sensitisation, positive skin prick test with a wheal diameter 3 mm or above, RAST test (or equivalent) grass-specific IgE class 2 or above.
Patients sensitive to tree pollens (n = 64) were treated in five clinics in Poland. Diagnosis and inclusion criteria were: male or female patients aged between 6-17 with seasonal symptoms due to birch, alder and hazel pollen sensitisation, positive skin prick test with a wheal diameter 3 mm or above, RAST test (or equivalent) birch, alder and hazel-specific IgE class 2 or above. Children and adolescents included in this study suffered from allergic rhinitis (and/or asthma) or allergic conjunctivitis associated with rhinitis or asthma.
Vaccines for the tree pollen-allergic patients were formulated with allergoids (glutaraldehyde-modified extracts) from three tree pollens (birch, alder, hazel). The tree pollen allergoids were adsorbed onto L-tyrosine depot adjuvant as a 2 % suspension. Vaccines for the grass pollen-allergic patients were formulated with allergoids produced in a similar manner from a 12 grasses and rye pollen mixture and adsorbed to L-tyrosine. In total, 90 patients were included and each received 3 increasing subcutaneous doses (300, 800, 2000 standardised units [SU]) in weekly intervals and a further top dose (2000 SU) at a 1-4 weeks interval. Each injection was 1.0 ml and contained 50 μg MPL® adjuvant. These injections were administered before the respective pollen seasons.
The symptom scoring scheme required patients to score their organ-related allergic symptoms for the eyes, nose and lungs according to severity (none = 1, mild = 2, moderate = 3, severe = 4). The points were totalled for each patient, providing a maximum possible score of 12. The medication score was devised by a generalised quantification of the medication intake (no medication = 1, occasional = 2, frequent = 3, regular = 4), to provide a maximum score of 4. The scores were taken at two timepoints: the previous pollen season (baseline investigation) and during the pollen season after therapy.
Titrated skin testing to identify threshold reactivity was performed at three time points: before therapy and two weeks after therapy (both before the pollen season) and then finally after the pollen season. Seven 1:3 serial dilutions of allergen were tested on both forearms using either a grass pollen mixture or a birch pollen extract. Histamine and allergen diluent served as positive and negative controls respectively. The diluent contained glycerol with phosphate buffer and phenol preservative. Wheals were measured with a computerised planimetry system (Summagraphics Bitpad and Digit computer programme). At the same time points, blood samples were taken for determination of specific immunoglobulins (AlaSTAT, Diagnostic Products Corporation). Tolerability was documented regarding types of local adverse events: redness/swelling (sub-divided into sizes of area), pain, itching, types of systemic reactions and any other adverse events.
All data were analysed by an independent statistician.
RESULTS
Patient population
Table I provides a summary of the patient demographics.
Clinical efficacy assessment
All statistical significances were calculated using the Wilcoxon signed rank test.
Symptom scores: patients scored their organ related symptoms for the first pollen season (baseline investigation, before therapy) and after therapy (second pollen season). Symptom scores are displayed in figures 1 and 2; significant improvements were found from both groups of patients.
Figure 1.--Total symptom score from grass pollen-allergic patients (n = 23, p < 0.01). Dispersion bars indicate standard deviation from the mean values.
Figure 2.--Total symptom score from tree pollen-allergic patients (n = 63, p < 0.01). Dispersion bars indicate standard deviation from the mean values.
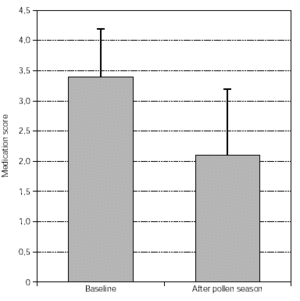
Medication scores: the intake of antiallergic symptomatic medication was scored and this is summarised in figures 3 and 4. Again, a significant improvement was seen from both groups of patients.
Figure 3.--Medication scores from grass pollen-allergic patients (n = 23, p < 0.01). Dispersion bars indicate standard deviation from the mean values.
Figure 4.--Medication scores from tree pollen-allergic patients (n = 63, p < 0.01). Dispersion bars indicate standard deviation from the mean values.
Titrated skin prick test
The skin prick test results for grass pollen-allergic patients are summarised in figure 5 and those from tree pollen-allergic patients in figure 6. Compared to baseline, both groups displayed a clearly significant reduction in skin reactivity, which was maintained throughout the pollen season.
Figure 5.--Titrated skin prick tests of grass pollen-allergic patients. N = 18, p = 0.035 (after therapy: baseline), p = 0.003 (after pollen season: baseline). Dispersion bars indicate standard deviation from the mean values.
Figure 6.--Titrated skin prick tests of tree pollen-allergic patients. N = 62, p < 0.001 (after therapy: baseline), p < 0.001 (after pollen season: baseline). Dispersion bars indicate standard deviation from the mean values.
Specific immunoglobulins
Measurement of specific IgE (trees, grasses) showed no change after treatment but there was a slight increase during the pollen season in both groups (data not shown).
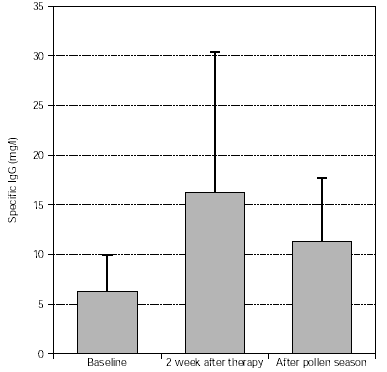
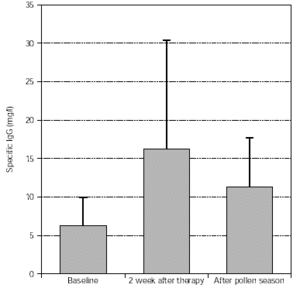
Levels of specific IgG (trees, grasses) significantly increased after treatment and this was maintained during the pollen season, as shown in figures 7 and 8.
Figure 7.--Specific IgG antibody response of grass pollen-allergic patients. N = 21, p < 0.001 (after therapy: baseline), p < 0.001 (after pollen season: baseline). Dispersion bars indicate standard deviation from the mean values.
Figure 8.--Specific IgG antibody response of tree pollen-allergic patients. N = 57, p = 0.003 (after therapy:baseline), p < 0.001 (after pollen season: baseline). Dispersion bars indicate standard deviation from the mean values.
Tolerability: adverse events
Local reaction rates are given in table II (grass/rye pollen SIT) and table III (tree pollen SIT). The grass/ rye vaccine was used for 103 injections, and following this administration 35 local reactions occurred (rate = 34 %). The tree pollen vaccine was used for a total of 256 injections, which were followed by 58 local reactions (rate = 23 %). A small number of patients received 5 or 6 injections. These were split volume doses, following the clinician's opinion to be most appropriate for highly sensitised patients or in response to a previous local reaction. Combining the data for both vaccines, the overall number of injections given was 359 and the overall rate of local reactions per injection was 25 %. Following the final (repeat) dose 4 (2000 SU), the event rate roughly halved from that of the previous dose in both types of vaccine (see tables II and III).
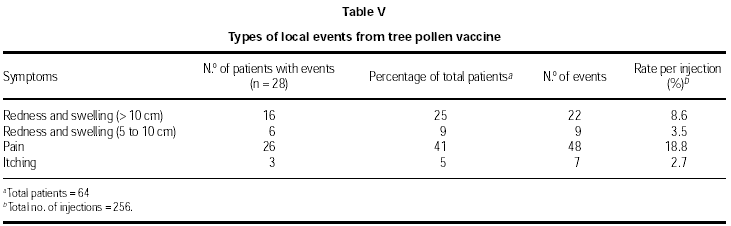
An analysis of the types of local events is shown in tables IV (grass/rye pollen SIT) and V (tree pollen SIT). A total of 13 and 28 patients respectively experienced local events.
Patients treated with the grass/rye pollen vaccine displayed a lower rate of redness for the highest area size (> 10 cm, 8 %) compared to the tree pollen patients (> 10 cm, 25 %). However, regarding redness ranging from 5 to 10 cm, the grass pollen patients showed a 27 % rate compared to a 9 % rate found with the tree pollen patients.
The statistic of event rates per injection also reflects the most frequent local events. These were: grass/rye pollen SIT, redness 5-10 cm (20.4 %), tree pollen SIT, itching (18.8 %).
Some pain was observed at the injection site of the tree pollen vaccine (41 %) and this was at a higher incidence than that found with the grass/rye pollen group (15 %). More itching (31 %) was reported by the grass pollen patients than the tree pollen patients (5 %).
No systemic reactions were documented from any of the patients treated with the grasses/rye vaccine. After injection of the tree pollen vaccine, systemic reactions (conjunctivitis, rhinitis, headache, breathing difficulties, pruritus) were observed following 8/256 injections, (rate = 3.1 %). These systemic reactions were reported from only one of the five clinics using the tree pollen vaccine. No systemic reactions were observed resulting from the high doses (3rd and 4th) at 2000 SU. Regarding the total number of injections (359), the total incidence of systemic reactions was 2.2 %.
Other adverse events
Other adverse events were documented in the post injection period. These were categorised as unlikely to be related to therapy, and were reported using WHO adverse reaction terminology. Events observed in 6/26 grass/rye pollen vaccine patients were: respiratory system (asthma, rhinitis), 3; vision disorders (conjunctivitis), 3; resistance mechanism disorders (otitis media), 1. Events observed in 23/64 tree pollen patients were: respiratory disorders, 17; vision disorders, 9; body as a whole, 7; central and peripheral nervous system, 1.
DISCUSSION
Children and adolescents were administered a short-term SIT using 4 injections of an allergoid/L-tyrosine vaccine incorporating the Th1 adjuvant monophosphoryl lipid A (MPL®) using grass/rye or tree pollen allergoid formulations (Pollinex® Quattro grasses/rye, trees,). Treatment with both vaccine formulations resulted in significant reductions in allergic symptoms and usage of anti-allergic medication. These findings are consistent with data from placebo-controlled double-blind studies in adult patients5,6. Local reactions to both tree and grass/rye pollen vaccines were also found comparable to that found in these adult studies. Systemic reactions were not detected in the grass pollen vaccine group, and only mild events were observed in the tree pollen vaccine group. Again, these results compare well with those found with adults.
A reduction in skin prick test sensitivity after treatment and after the pollen season was seen with both groups, which was a marker result consistent with findings in other studies involving this short-term therapy5,6. Another well-recognised marker, the specific IgG antibody response, increased after therapy and after the pollen season in comparison to the baseline. Following therapy, little change in specific IgE was seen in both groups, which was again consistent with that previously found with this vaccine in the treatment of adult patients5,6. This result is probably due to the Th1-inducing activity of MPL® adjuvant, which would bias cytokine activities to discourage an IgE response4.
The WHO Position Paper on allergen immunotherapy has stated that more studies are needed to determine how immunotherapy may modify the allergic disease in children7. So far, although we have seen some high quality studies8, the number of publications on this topic is comparatively low. At the same time, researchers are suggesting that there is a real need for allergy treatment strategies such as immunotherapy for children13. Data from this open study shows that regarding both efficacy and safety, the results are similar to those found in controlled studies. Effective immunotherapy may significantly improve the quality of life for a child over the critical period to adulthood. Additionally, health authorities should note that there is also a potential pharmacoeconomic effect14.
In summary, these results have paralleled outcomes from previously reported double-blind, placebo-controlled studies in adults, demonstrating a new treatment for children and adolescents with respiratory allergy to grass and tree pollens which provides clinical and immunological efficacy with good tolerability.
ACKNOWLEDGEMENTS
The following clinicians recruited grass/rye pollen-allergic patients for the study in Portugal:
Dr. M.A. Aguilar (Coimbra), Dr. H. Costa (Gaia), Dr. J.P. da Fonseca Moreira da Silva (Porto), Dr. P. Lopes de Mata (Lisboa), Prof. M. Oueiros (Porto).
The following clinicians recruited tree pollen-allergic patients for the study in Poland:
Prof. D. Chmielewska (Warszawa), Prof. P. Kuna (Zgierz), Prof. J. Maloplepszy (Wroclaw), Prof. B. Romanski (Bydgoszcz), Prof. E. Zawisza (Warszawa).
MPL® is a registered trademark of the Corixa Corporation, Seattle, WA, USA.