INTRODUCTION
Asticot is a blowfly larvae commonly used as bait by anglers. Blowflies (Family Calliphoridae) are scavengers which deposit their eggs on rotting meat, fish, garbage, fecal matter or dead animals. Inside the egg, a larvae will develop and when it is ready to leave the egg, it will wriggle out for further maturation and will use the carrion as its food source. This larvae develops quickly and then crawls a short distance away to burrow into the soil. Once under the soil, the maggot pupates, with adult flies emerging in a few days. These maggots are almost identical and it is so difficult to distinguish that the name Asticot is used without worrying about the exact species concerned. Allowing the larvae to reach its adult stage is the only way of identifying them, but it is still difficult since the adults are also similar.
Allergic reactions have been described through emanations or in handling of Asticot1, Lucilia Caesar (greenbottle)2,3 and Calliphora (bluebottle)4-6 maggots. Sensitization may occur in occupationally exposed workers or in amateur fishermen with occasional exposure
IgE mediated hypersensitivity to unknown allergens of fly larvae have been reported. As far as we know there are no previous reports in the literature that describe the characterization of allergens in Asticot maggots.
CASE REPORT
We report a case of a 12 year old male, an amateur fisherman, referred to our allergy department with episodes of nasal obstruction, rinorrhea, paroxysmal sneezing and facial angioedema immediatly after he started fishing, and which disappeared a few hours later at home. He developed symptoms when handling Asticot maggot used as fishing bait, inserting it on the fishhook. He had no family history of atopic diseases and he suffered from mild persistent allergic rhinitis in the last three years due to Alternaria. At initial evaluation, clinical examination was normal, hematologic and biochemical tests revealed no abnormalities. Skin prick tests (SPT) with a battery of commercially available common inhalants revealed positive immediate reaction to Alternaria.
Clinical and immunological studies were performed in order to confirm a hypersensitivity mechanism to Asticot larvae and the allergens involved in this case.
MATERIALS AND METHODS
Preparation of Asticot extracts for in vivo and in vitro tests
Commercially available Asticot species were extracted in phosfate-buffered saline (PBS) by mixing 4g in 40 ml of PBS (pH 7.3) at laboratory temperature. After it had been stirred for 30 min, the solution was centrifuged at 3000 g for 10 min. The supernatant was centrifuged again, and the solution then passed through a Millipore filter (0,20 μ) for sterilization (Millipore, Molsheim, France) with a final dilution of 1/10 w/v.
The protein concentration of the final extract as determined by turbidimetric analysis (Boeringer, Germany) was 1.4 mg/ml
Skin Prick Test
Skin prick tests (SPT) were performed with the allergy pricker lancet (Dome-Hollister-Stier) as described elsewhere7. Histamine phosphate (10mg/ml) served as positive control and NaCl (0,9 %) as negative control. The tests were read after 20 minutes. A reaction was considered to be positive if the wheal size was at least 3-mm diameter larger than negative control.
SPT were done with our own Asticot extract. Ten atopic and ten non-atopic controls were tested for SPT with Asticot extract. Atopics were defined as having a positive clinical history and a SPT reaction more than 3 mm to at least one common allergen. A prick-by-prick test was also performed with live asticot bait.
Specific IgE determinations
Specific IgE againts Asticot extract was determined by ELISA as described elsewere8. Briefly, protein extracts (5 μg/ml) were bound to 96-well polystyrene microplates (Costar, Cambridge, Mass.) by overnight incubation at 4° C. After washing with PBS-0.1 % Tween, plates were neutralized with 1 % bovine serum albumin (1 hour at room temperature), washed, and incubated with undiluted serum samples (4 hours at room temperature). Plates were washed and incubated with 1:1000 dilution of goat antibody to human IgE (Biosource international. Camarillo, CA, USA). After a final washing, a substrate solution was added and the color was allowed to develop. The enzymatic reaction was stopped with HCl, and the absorbance values were measured at 492 nm. Results were considered positive when above the mean + 3 SD of a control group (10 non-atopic patients).
SDS-PAGE and Immunoblotting
Asticot extracts were diluted in 0.1 mol/L Tris HCl, 2 % sodium dodecyl sulfate, 10 % glycerol, 0.1 % DTT and 0.01 % bromophenol blue. Gel electrophoresis of Asticot extract, was carried out in 12 % gradient polyacrylamide gels (Biowhittaker Molecular Applications (BMA). Rockland ME. USA) in both reducing and non-reducing conditions according to Laemli9, using a Hoefer mini VE Unit (Amersham Pharmacia Biotech, Hoefer Scientific Instruments, San Francisco, CA, USA). Approximately, 40 μg of protein were applied to each lane. Molecular weight was estimated with commercial protein standards (Bio-Rad, Hercules, CA, USA). After the separation, one set of proteins was stained with Coomasie brilliant blue (Bio-Rad) and another set was transferred by electroblotting into Hybond-P. PVDF membrane (Amersham Pharmacia Biotech), following the method of Towbin10. Unreacted membrane sites were blocked for 1h with TBS containing 3 % bovine serum albumin and 0.1 % Tween 20 (TBS-T-BSA). The membranes were then incubated for 1h at room temperature with patient's or control's serum diluted 1/20. Washes were performed in TBS 0.1 % Tween. Afterwards, alkaline phosphatase-conjugated goat anti-human IgE (Biosource international. Camarillo, CA, USA), 1/200 in TBS-T-BSA, was incubated for 1h at room temperature. Finally, the reaction was developed with nitro blue tetrazolium 5-bromo-4cloro-3indolyl phosphate (NBT-BCIP) as substrate.
Specific nasal challenge test with Asticot extract
Specific nasal challenge test was performed using a Rhinospir 164 (Sibel) rhinomanometer.
The patient was not exposed to Asticot maggots for one week previous to the challenge test, asymptomatic and he did not take any medication that might affect the test results.
SPT with ten-fold dilutions of Asticot extracts were previously performed to determine the safety of the procedure before the nasal challenge test. The starting dose was that corresponding to a SPT with a 2-3 mm wheal diameter. A control challenge with PBS was carried out before the antigen challenge. When the patient demonstrated a less than 50 % increase of nasal airway resistance with PBS, he was considered suitable for the challenge. The patient inhaled aerosolized Asticot extract in progressively increasing concentrations at 20 minutes interval with active anterior rhinomanometry control measures. Positive responses were defined as > 100 % increase in nasal airways resistances from baseline calculated at fixes pressures of 150 Pa according to the European Committee on Standardization of rhinomanometry11.
RESULTS
Prick-by-prick test with live asticot bait was positive with 20-mm wheal and 50 mm erythema.
Prick test with our own aqueus Asticot extract showed an immediate positive reaction with 15-mm wheal and 40 mm erythema. Negative responses were observed in ten atopic and ten non-atopic controls.
Specific IgE antibodies against Asticot as determined by ELISA were found in the patient's serum. IgE activity were not detectable in serum samples from 4 healthy patients
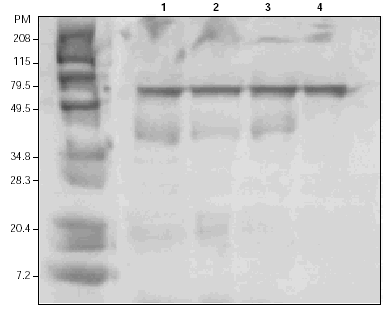
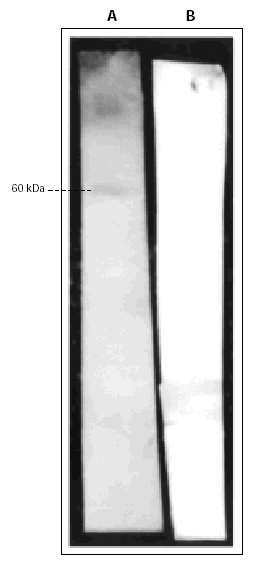
Coomasie-blue staining after SDS-PAGE separation of the extract showed three main antigens with an approximated MW of 60, 40, and 30 kDa (fig. 1). Other sets of proteins were subjected to IgE Western Blotting which identified specific reactivity toward one antigenic band with approximately MW of 60 kDa, not recognized by the secondary antibody (fig. 2).
Figure 1.--Protein separation by 12 % gradient SDS-PAGE and Coomasie blue staining of the gel. Left lane, molecular weight standards (in kDa). Lanes 1,2,3: Asticot extract with β-mercaptoethanol. Lane 4: Asticot extract in the absence of β-mercaptoethanol.
Figure 2.--IgE-Immunoblotting of Asticot extract (40 μg/lane) after protein transfer to PVDF membranes. The Membranes were incubated with patient's serum (Lane A) or secondary antibody alone, goat anti-human IgE (Lane B).
The specific nasal challenge test with Asticot extract at 1/10 w/v concentration, elicited an immediate clinical (nasal obstruction, hydrorrea) and rhinomanometric response with a 200 % increase in nasal resistances.
DISCUSSION
In 1982, Stockley et al reported the first case of hypersensitivity to fly larvae belonging to Calliphoridae family in a fisherman with contact urticaria and late asthmatic responses associated with handling this maggot4. A circulating immune complex disease was suspected by the presence of specific IgG antibodies to whole larvae. In 1987, Sestin et al describe dual and late asthmatic responses in two patients after exposure to larvae of calliphora erytrocephala used as fishing bait, but skin test and RAST studies confirmed an IgE-mediated hypersensitivity mechanism5. Siracusa et al demonstrated that emanations from live fish baits are sensitizers, which have the potential to elicit IgE-mediated asthma in anglers or in occupationally exposed workers2,3. Early and late asthmatic reactions were observed, beginning up to 16 hours after exposure to these baits, which makes it difficult for the identification of insect antigens as the causal agent of allergic asthma. The Lucilia Caesar (Family Calliphoridae) larvae was the main allergen responsible for symptoms in all but one patient in this study.
Protein contact dermatitis has been recently reported in a fisherman with cronic dermatitis of his hands during the summer, while handling flesh fly maggots12.
Other live fish baits have been implicated in allergic reactions. Skin tests and RAST studies confirmed that reactions were due to specific IgE-mediated hypersensitivity. Insect larvae like those of the bee moth (Galleria Mellonela)13,14 and the mealworm (Tenebrio Mollitor)3,15 have been described as ethiological agents of occupational asthma. There are also some worms such as the marine worm Marphisa Sanguinea16 and earthworms (Eisenia Foetida17 and Lumbricus terrestris18) reported to cause cutaneous and respiratory hypersensitivity reactions.
The blowfly, seems to be a more potent sensitizer than other insects larvae used as baits3. In most published cases, allergic reactions to these maggots were due to occasional exposure in anglers during fishing season. No allergenic cross-reactivity, as determined by RAST inhibition assays, were observed between fly larvae and other insect larvae used as baits3,15.
Asticot maggots have not been cited in literature until 1999 by González et al1. In this case of rhinoconjunctivitis and asthma provoked by Asticot maggots in an amateur fisherman, only SPT with asticot extract was performed showing an immediate positive response that confirms a hypersensitivity mechanism.
The clinical picture of our patient, with nasal symptoms shortly after exposure to Asticot maggots, strongly suggests hypersensitivity to this fish bait. The positive specific nasal challenge test confirmed Asticot was the ethiological agent in this case. The positive immediate response in SPT and the demonstration of specific serum anti-Asticot IgE by ELISA and Immunoblot, strongly suggest the underlying type I hypersensitivity mechanism. The negative results in skin prick tests in a group of unexposed normal and atopic subjects and a negative response in Western Blot to secondary antibodies alone, confirm the specificity of these findings. IgE reactivity was preserved with reduced and non-reduced antigen, as well as after denaturation, suggesting that the involved epitopes are not conformational nor dependent on SH2 groups, and that they are likely encoded in the amino-acid primary sequence.
As far as we know there are no previous published reports that describe the allergens involved in allergic reactions to Asticot maggots. We report the first characterization of allergens in this fly larvae and we demonstrate a 60 kDa protein as the main allergen in this case.
Angling is a sport and hobby for many people. There are several types of live fish baits, but in Spain, the asticot maggot and the common earthworm are the most important ones used for angling. We have shown other cases of respiratory reactions related to fishing activity and we are of the opinion that epidemiologic studies need to be performed in order to know real importance of sensitization to these Asticot maggots in exposed patients.
Asticot maggots have to be taken into account as a posible causative agent of respiratory allergic reactions in anglers who are exposed to emanations of these live fish baits.