Comparison of the number of mast cells in the active stage and that in remission in the same patients with ulcerative colitis with immunohistochemical staining remains to be elucidated, and analysis of the number of mast cells in benign and malignant colonic lesions is insufficient.
MethodsUsing immunohistochemical methods, morphological examinations of mast cells were undertaken in colonic tissues from 8 patients with ulcerative colitis and 10 patients with colonic primary cancer, which were formalin-fixed and paraffin-embedded. Changes in the number of mast cells in the active stage and in remission in the same patients with ulcerative colitis were investigated. Then, the number of mast cells in malignant tissues and adjacent healthy tissues obtained from the same patients with colonic primary cancer were compared, and finally the number of mast cells was compared among the samples from benign and malignant colonic lesions.
ResultsAccumulation of mast cells was found to be significant in the active stage of ulcerative colitis compared with remission in the same patients. The number of mast cells in colonic primary cancer was significantly increased compared with that in adjacent healthy tissues. The number of mast cells in ulcerative colitis was significantly greater than that in adjacent healthy tissues from patients with colonic primary cancer, irrespective of the stages of ulcerative colitis.
ConclusionsWe were the first to analyse mast cells in the active stage and in remission in the same patients with ulcerative colitis using immunohistochemical methods, and compared the number of mast cells between benign and malignant colonic lesions.
It has been reported that mast cells may be involved in the health and disease of the intestine1,2. Since their first recognition by Ehrlich in 1878, mast cells have often been observed at different sites of neoplasm. Simu and Csaba3 described an increased number of mast cells in tumour-bearing patients including colonic cáncer. However, Svennevig et al4 reported the number of mast cells in the peritumoural stroma did not exceed the number of mast cells in normal colonic wall, and Lachter et al5 demonstrated that the number of mast cells was lower in colorectal carcinoma than in normal specimens. On the other hand, many authors identified changes in mast cells in inflammatory bowel diseases including ulcerative colitis. However, contradicting data have also been reported regarding the distribution of mast cells in the intestinal mucosa of patients with ulcerative colitis, namely the number was increased in some studies6,7, unchanged8, and decreased in others9. One reason for the discrepancy in mast cell distribution in malignant and benign colonic lesions may be due to differences in the methods used for tissue preparation, such as tissue fixation, staining techniques and methods of cell counting as described by Craig et al10. In fact, conventional staining methods are inferior to the sensitive immunohistochemical techniques that use antibodies to specific mast cell proteinase such as tryptase, and some immunohistochemical studies have noted that one of the features of ulcerative colitis among inflammatory bowel diseases may be a marked increase of mast cells in the affected mucosa11,12. However, to the best of our knowledge in the literature there has been no report that investigated the number of mast cells in the active stage and in remission in the same patients with ulcerative colitis using immunohistochemical staining for human mast cell tryptase.
Very recently, we have reported a case of eosinophilic colitis that showed an accumulation of mast cells in the colonic lesions using immunohistochemical staining13. Moreover, in the recent study of mast cells in benign and malignant breast lesions from our laboratory14, we demonstrated that the number of mast cells was significantly greater in malignant lesions than that in benign lesions of the breast. We have expanded our studies and, in this paper, we first investigated the changes in the number of mast cells in the active stage of ulcerative colitis and in remission of the disease in the same patients, and present that an accumulation of mast cells was found to be significant in the active stage compared with remission of ulcerative colitis. Secondly, we studied the number of mast cells in colonic primary cancer and present that the number was significantly increased compared with that in adjacent healthy tissues. Finally, our research has brought us to the realization that the number of mast cells in ulcerative colitis is significantly greater than that in adjacent healthy tissues from the patients with colonic cancer irrespective of the stages of ulcerative colitis.
MATERIALS AND METHODSSubjectsEighteen patients with histologically confirmed ulcerative colitis or colonic cancer were enrolled in the study. Biopsy specimens were obtained from eight patients with active ulcerative colitis (3 males and 5 females) and malignant tissues were surgically obtained from ten patients with colonic primary cancer (5 males and 5 females) at Shin-Ohra Hospital, Gunma, Japan during a three-year period. Biopsy specimens were also obtained from each patient with ulcerative colitis when it became in remission after treatment with prednisolone. In patients with colonic primary cancer, adjacent healthy tissues were taken from the cancer-free resection edges at least eight cm from the tumour. Median ages of the patients with ulcerative colitis and with colonic primary cancer were 56.8years (range 27–75years) and 71.0years (range 62–82years) respectively.
None of the patients with colonic primary cancer had been receiving chemotherapy or radiation therapy before surgery. Tumour grading and staging was performed according to the method proposed by the American Joint Committee for Cancer Staging15. All of the patients investigated in this study had tumours in stage II. The clinical diagnosis of ulcerative colitis was based on an appropriate history, clinical examination, endoscopic findings and radiological criteria16. The clinical stage of the disease was determined by clinical symptoms and signs, and by the endoscopic appearances and histology, as shown below.
The Institutional Ethics Committee of Gunma Institute for Allergy and Asthma, Shin-Ohra Hospital approved the present study, and written informed consent was obtained from each individual before the study commenced.
Processing of Tissue SpecimenTissue specimens were fixed with 10 % neutral formalin, and then subsequently embedded in paraffin to prepare 4-μm thick sections. The serial sections were placed on poly-L-lysine-coated glass slides followed by air-dried over a period of at least two hours. Each material was stained with haematoxylin and eosin on routine histological examination.
HistologyRoutine pathology reports were used to access benign and malignant histological types. In the active stage of ulcerative colitis, in addition to erosion and ulceration of the lining epithelium of the mucosa, the mucous membrane was inflamed and its capillary blood vessels were dilated and engorged. The epithelium of the crypts was depleted of goblet cells, and crypt abscess formation was observed. There was an inflammatory cell infiltration such as lymphocytes and plasma cells in the lamina propria. In remission, the mucous membrane became atrophic, and the epithelial glands were found to be irregular and short in the same patients. There was also an accumulation of fibrotic tissue between the mucosa and the muscularis mucosa in remission.
ImmunohistochemistryAn immunohistochemical method described by Irani et al17 was used for the detection of mast cells. In brief, sections were dewaxed in xylene and rehydrated with a graded alcohol series. Endogenous peroxidase in the tissues was inhibited by incubation in methanol with 0.6 % hydrogen peroxide for 30min at room temperature as originally described17. Serial sections were incubated in a humid chamber overnight at 4°C with a monoclonal murine anti-tryptase antibody (Chemicon International, Temecula, CA, USA: 0.7μg/ml) diluted 1:400 in Tris buffer (50mM Tris–HCl buffer, pH 7.6) containing 1 % bovine serum albumin. The sections were then washed extensively in running TTBS buffer (50mM Tris–HCl, 154mM NaCl, 0.05 % Tween-20, pH 7.4) at 4°C for 10sec, followed by washing extensively in TTBS buffer for 15min at room temperature (with 3 changes of buffer), and washing in distilled water for 5min. As described by Cordell et al18, sections were incubated for 40min in a humid chamber at room temperature with a rabbit anti-mouse immunoglobulin antibody (Dako Co., Carpinteria, CA, USA) diluted 1:100 with Tris buffer followed by washing in TTBS buffer for 15min at room temperature (with 3 changes of buffer) and washing in distilled water for 5min. Sections were then incubated for 40min in a humid chamber at room temperature with a monoclonal mouse anti-alkaline phosphatase antibody conjugated to alkaline phosphatase (APAAP complex; Dako) diluted 1:50 in Tris buffer as described18. The sections were washed extensively in running TTBS buffer at 4°C for 10sec, then washed in TTBS buffer for 15min at room temperature (with 3 changes of buffer), and washed in distilled water for 5min. Colour was developed for 10min at room temperature with a fast red substrate containing naphthol AS-MX phosphate (Dako) (100mM Tris–HCl buffer, pH 8.2, containing naphthol phosphate, fast red and levamisole). The sections were washed with distilled water, counterstained with Mayer's hematoxylin for 5min, and then washed in running water for 10min and mounted in glycerol-gelatin (Sigma Chemical Co., St. Louis, MO, USA). Photomicrographs were taken with an Olympus PM-10AK and an Olympus BX-50 microscope.
Mast cells were counted under 100-x magnification, using the microscope equipped with a 0.1 × 0.1mm ocular grid (Olympus Eyepiece Micrometer U-OCMSQ 10/10) by an investigator who was blinded to the clinical diagnosis of each subject. Of biopsy specimens from the patients with ulcerative colitis, we examined only those sections, which showed lamina propria was clearly visible under the microscope. Care was taken to exclude areas which showed excessive oedema of the lamina propria and uneven distribution of the glands due to excessive gland atrophy. The lymphoid follicles themselves and the lamina propria adjacent to erosion or ulcer were excluded from the examination. With regard to colonic cancer, we counted the number of mast cells near the tumour margin up to no more than 1mm away from the first mast cell detected; necrotic foci present within central portions were excluded due to its low number of mast cells. Mast cells were counted in 10 neighbouring grid fields, and the mean of the best 6 grid fields in each section was then analysed. Cells showing an equivocal staining or lacking a nucleus were not counted. Numbers were normalised per 1mm2.
Statistical AnalysisData were analysed with non-parametric Kruskall-Wallis and Mann-Whitney tests. Collated specimens were analysed with Wilcoxon test for non-parametric data. Differences with p < 0.05 were considered to be significant.
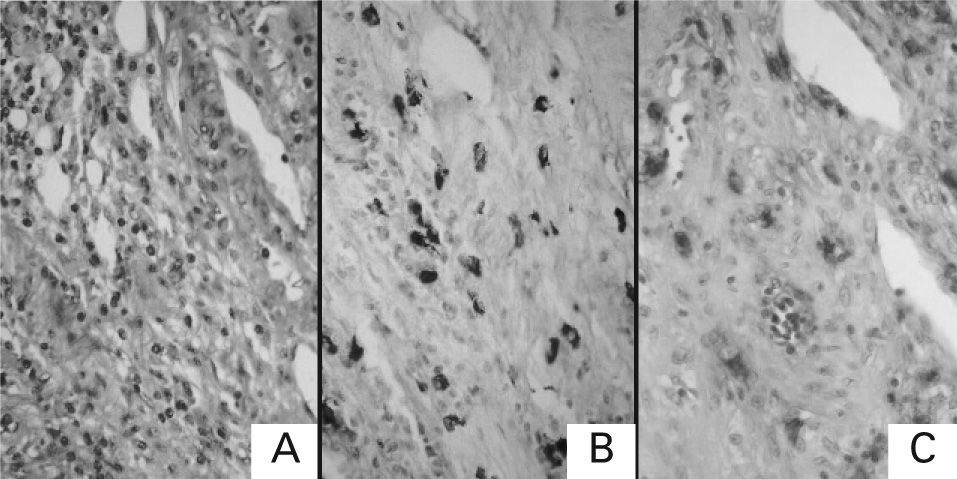
RESULTSHaematoxylin-Eosin and Toluidine Blue StainingTissue sections were stained by routine haematoxylin-eosin processing, and, histochemical staining of mast cells with 0.1 % toluidine blue (pH 0.5) was performed to exclude the possibility of false positive in the immunohistochemical staining of each sample. Figure 1 shows the staining patterns of malignant lesion from a patient with colonic primary cancer with each staining (A: haematoxylin-eosin staining; B: toluidine blue staining; C: immunohistochemical staining for tryptase). Mast cells stained histochemically with toluidine blue were identified by their characteristic metachromasia, and they were immunolabelled for tryptase.
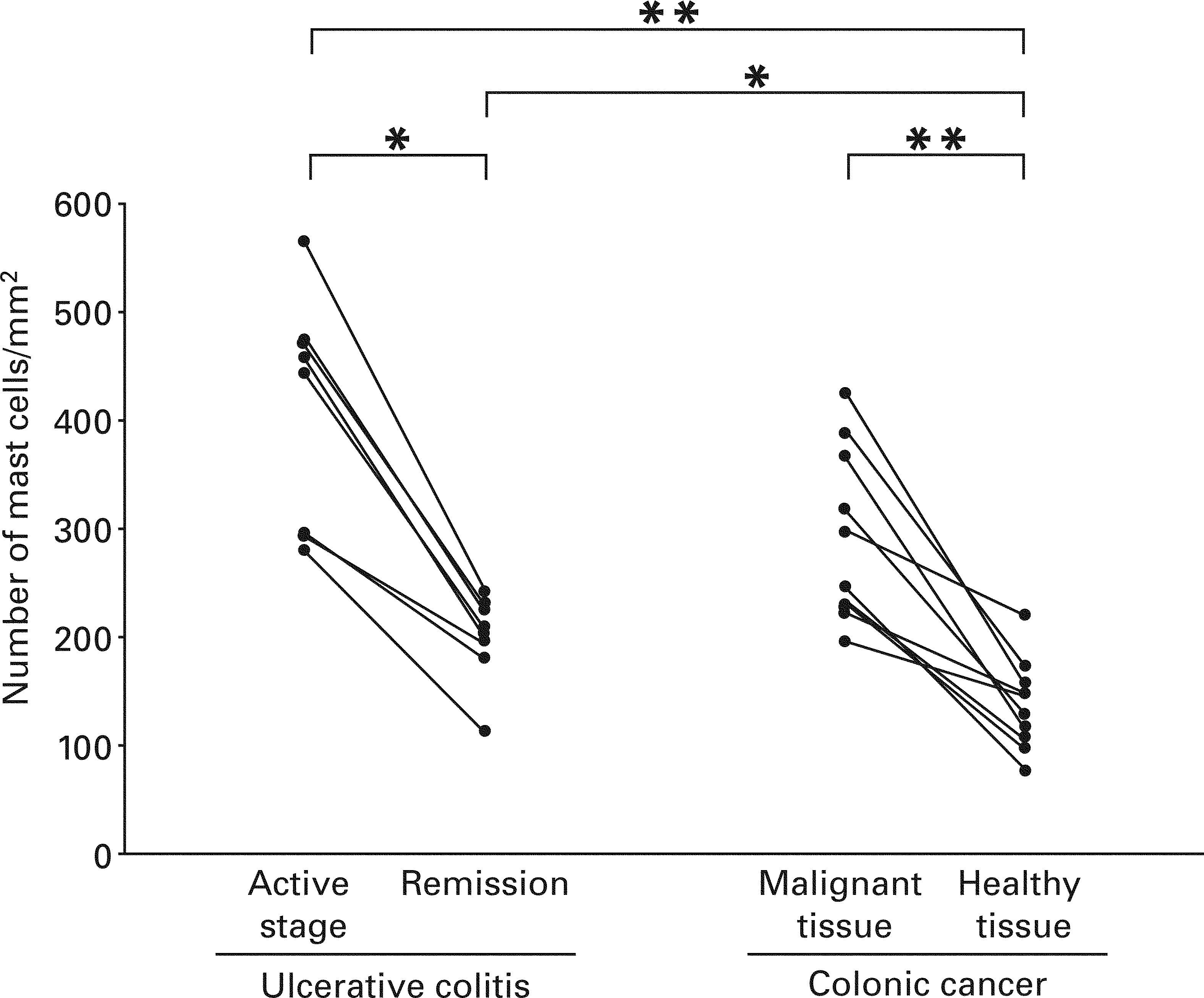
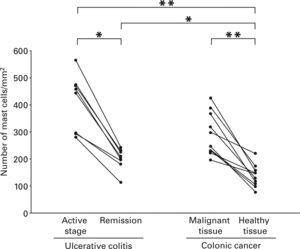
Mast Cells in Ulcerative ColitisEach lesion in the active stage of ulcerative colitis contained mast cells as shown in Figure 2. The median of the number of mast cells in the active stage of patients with the disease and that in remission of the same patients were 451.3/mm2 with a range from 280.5 to 566.3/mm2 and 206.1/mm2 with a range from 112.5 to 241.4/mm2 respectively, and mast cells were present more frequently in the active stage compared with remission in the same patients (p < 0.05) (Fig. 3).
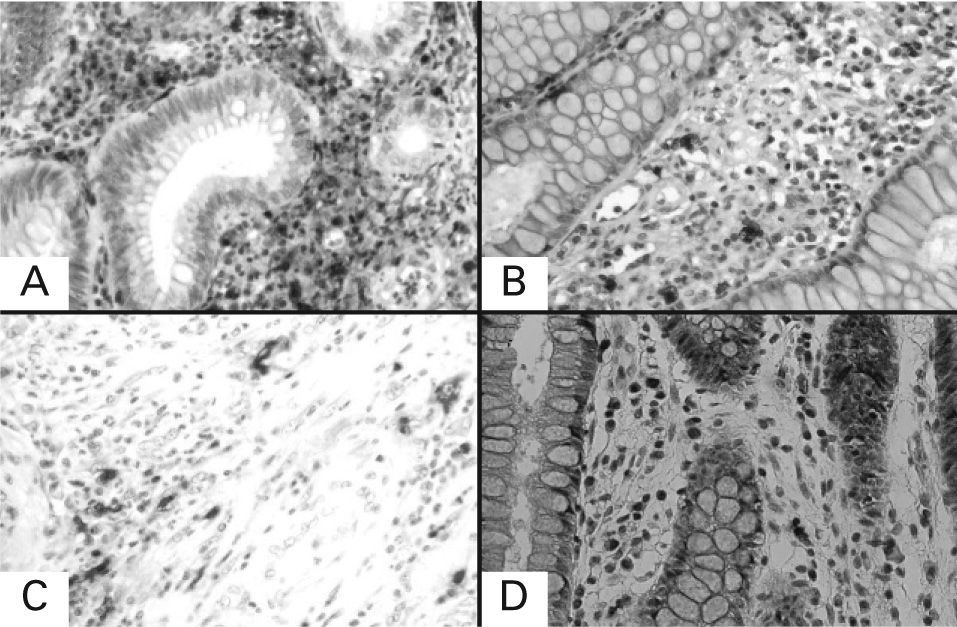
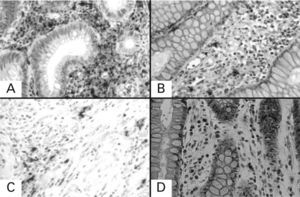
Immunohistochemical staining for tryptase of serial sections of the lesional tissue from patients with ulcerative colitis and colonic primary cancer. A: lesional tissue from patient with ulcerative colitis in active stage; B: lesional tissue from the same patient with ulcerative colitis in remission; C: malignant lesion from patient with colonic primary cancer; D: adjacent healthy tissue from the same patient with colonic primary cancer. Under 400× magnification.
Number of mast cells in the lesional tissue from patients with ulcerative colitis and colonic primary cancer. The number was compared between active stage and remission in the same patients with ulcerative colitis. In addition, the number was compared between malignant lesions and adjacent healthy tissues from the same patients with colonic primary cancer. Moreover, the number was compared among groups. Data were analysed with non-parametric Kruskall-Wallis and Mann–Whitney tests. Collated specimens were analysed with Wilcoxon test for non-parametric data. Statistical significance between the groups as indicated at *p < 0.05 and **p < 0.01, respectively.
Each malignant lesion from the patients with colonic primary cancer contained mast cells as shown in Figure 2. The median of the number of mast cells in malignant tissues and that in adjacent healthy tissues from the patients with colonic primary cancer were 271.4/mm2 with a range from 195.7 to 424.3/mm2 and 137.7/mm2 with a range from 75.3 to 220.6/mm2 respectively, and mast cells in colonic primary cancer were present more frequently than in adjacent healthy lesions from the same patients (p < 0.01) (Fig. 3).
Mast Cells in Benign and Malignant Colonic LesionsThe number of mast cells in ulcerative colitis was significantly greater than that in adjacent healthy tissues from the patients with colonic primary cancer, irrespective of the stages of ulcerative colitis as shown in Figure 3 (active stage at p < 0.01 and remission at p < 0.05, respectively).
DISCUSSIONMany authors identified changes in mast cells in begin and malignant colonic diseases including ulcerative colitis and colonic cancer1–7,9,11,12. However, contradicting data have been reported regarding the distribution of mast cells in the intestinal mucosa of the diseases. We may easily speculate that one reason for the discrepancy in mast cell distribution in begin and malignant colonic lesions was due to differences in the methods used for tissue preparation, such as tissue fixation, staining techniques and methods of cell counting10. However, we are not aware of any report with immunohistochemical staining which indicates differences in the number of mast cells in the active stage and in remission in the same patients with ulcerative colitis, and the difference between the number of mast cells in begin and malignant colonic lesions remains to be unknown.
As the first part of study, we undertook a morphological study of mast cells in ulcerative colitis. The range of the number of mast cells in our study was in agreement with that reported by others8,12,19. We demonstrated that the number of mast cells was significantly increased in the active stage of ulcerative colitis compared with that in remission in the same patients.
Next, we undertook a morphological study of mast cells in colonic primary cancer and found that the number of mast cells in malignant tissues from colonic primary cancer was significantly increased compared with that in adjacent healthy tissues, suggesting that mast cells may participate in colonic malignant lesions to play some regulatory roles. In this study, we were not able to investigate whether there was any relationship between the histological characteristics and the number of mast cells. In fact, we have already reported that the number of mast cells was significantly greater in scirrhous carcinoma than in papillotubular carcinoma of the breast, suggesting that the number of mast cells may decrease in parallel with their grade of differentiation14. However, the role of mast cells in colonic cancer is poorly defined and its role in host defence mechanisms has been the subject of controversy. Some investigators20–22 have found a negative correlation between infiltrating mast cells and prognosis; others23 have found a positive correlation. Further studies are required.
An interesting result in this study was that the number of mast cells in ulcerative colitis was significantly higher than that in adjacent healthy tissues from the patients with colonic primary cancer, irrespective of the stages of ulcerative colitis. Mast cells-derived mediators may be responsible for some of symptoms of ulcerative colitis6, and it has been reported that reduction in mast cells number in corticosteroid-treated patients may be an important mechanism of action of corticosteroids in inflammatory bowel disease24. All of the patients of ulcerative colitis in this study were treated with prednisolone and became in remission of the disease. Taking these reports into consideration, we are able to speculate that mast cells may participate in ulcerative colitis to play some regulatory roles.
In this study, we were not able to investigate whether there was any intraconversion of subtypes of mast cells during the disease processes. However, Tan et al23 reported that the percentage of mast cells positive for tryptase and the cells positive for tryptase and chymase was not different between colorectal cancer and normal control. Also the concentrations of these two mast cells in specimens of colon from patients with ulcerative colitis have been shown to be not different from normal control8,25.
To conclude, we were the first to analyse mast cells in the active stage and remission in the same patients with ulcerative colitis, and to compare the number of mast cells between benign and malignant colonic lesions using immunohistochemical methods. We found that mast cells were present more frequently in the active stage compared with remission in the same patients, and the number of mast cells in ulcerative colitis was significantly greater than that in adjacent healthy tissues from the patients with colonic primary cancer, irrespective of the stages of ulcerative colitis. These results suggest possible roles of mast cells in benign and malignant colonic diseases such as colonic cancer and ulcerative colitis. Further studies are required.