With the availability of high-quality asthma guidelines worldwide, one possible approach of developing a valid guideline, without re-working the evidence, already analysed by major guidelines, is the ADAPTE approach, as was used for the development of National Guidelines on asthma.
MethodsThe guidelines development group (GDG) covered a broad range of experts from medical specialities, primary care physicians and methodologists. The core group of the GDG searched the literature for asthma guidelines 2005 onward, and analysed the 11 best guidelines with AGREE-II to select three mother guidelines. Key clinical questions were formulated covering each step of the asthma management.
ResultsThe selected mother guidelines are British Thoracic Society (BTS), GINA and GEMA 2015. Responses to the questions were formulated according to the evidence in the mother guidelines. Recommendations or suggestions were made for asthma treatment in Mexico by the core group, and adjusted during several rounds of a Delphi process, taking into account: 1. Evidence; 2. Safety; 3. Cost; 4. Patient preference – all these set against the background of the local reality. Here the detailed analysis of the evidence present in BTS/GINA/GEMA sections on prevention and diagnosis in paediatric asthma are presented for three age-groups: children with asthma ≤5 years, 6–11 years and ≥12 years.
ConclusionsFor the prevention and diagnosis sections, applying the AGREE-II method is useful to develop a scientifically-sustained document, adjusted to the local reality per country, as is the Mexican Guideline on Asthma.
According to estimates of the World Health Organisation and the International Forum of Respiratory Societies, there are between 235 and 300 million people worldwide suffering from asthma1 and in Mexico it is calculated that 7% of the population has asthma.2,3 The General Directory for Health Information (DGIS, by its initials in Spanish) reported that asthma accounted for 20% of the hospitalisations for respiratory illnesses in 2013, with a 0.03% fatality rate.4 It has been observed that among the different institutes that provide medical care in Mexico, there is a lack of harmonisation regarding the management of patients with asthma. In general, there are still multiple aspects that are sensitive to improvement in the prevention, diagnosis and treatment of this disease in Mexico.
Therefore, the National Institute of Respiratory Diseases (INER, by its initials in Spanish), in a joint effort with the Mexican Society of Pulmonology and Thoracic Surgery and members of the Mexican College of Clinical Immunologists and Allergists (SMNyCT and CMICA, by their initials in Spanish) convened thirteen national professional societies to join efforts and develop a National Asthma Guideline. Aware of the high-quality guidelines that already exist worldwide on asthma, it was decided to use the ADAPTE approach5 to develop a transculturation of the fused evidence of three of the best and most recent asthma guidelines globally available. The objective of the Guía Mexicana del Asma (GUIMA) 2017 project was to develop an evidence-based, practical guideline on the prevention, diagnosis and treatment of asthma, including asthma exacerbations. The guideline moreover had to have a solid base among specialists and primary health care physicians throughout the country.
The herein presented paper represents the collective evidence of the three international guidelines, selected as ‘Mother’ guidelines, and their fused recommendations for the prevention and diagnosis of asthma in children, adapted to the Mexican reality by a group of 56 local experts and primary care doctors, representing 13 national medical societies and two methodologists. In a subsequent paper the evidence on asthma treatment in children shall be presented.
MethodsA core group, in charge of the coordination and content of the guideline was created, consisting of eight expert pulmonologists and allergists. Subsequently, presidents of 13 professional societies, whose members treat patients with asthma in Mexico, were invited to assign 3–5 of their members to be integrated into the broad Guideline Development Group (GDG), consisting of 50 physicians. Other members of the GDG were methodologists, respiratory therapists and a specialised nurse. In the course of 19 months, three face-to-face meetings were held, and the rest of the work, including the Delphi rounds, was done via electronic communication.
For the development of GUIMA 2017 we followed the ADAPTE approach for transculturation of guidelines.5 Briefly, first the SCOPE document was created, explicitly defining the scope of the guideline. It was decided the analysis would include some aspects of asthma prevention, and an in-depth review of asthma diagnosis, treatment and diagnosis and treatment of the asthma attack, in both children and adults. After defining the scope, a literature search was conducted for asthma guidelines, yielding a total of 40 guidelines. The quality of the 11 most prominent and recent ones was evaluated with AGREE-II by two members of the core-team per guideline.6 Finally, the best three guidelines were selected: the British Thoracic Society Asthma Guideline (BTS) 2014,7 the Global Initiative on Asthma (GINA) 2015,8 and its update in 20169 and the Guía Española del Manejo del Asma (GEMA) 2015.10 These function as the ‘mother’ guidelines, from where the evidence is recollected to make GUIMA.
The next step was to develop the critical route of the asthma management process, for primary, secondary and tertiary asthma health care. After that the core group developed clinical questions, to cover each phase of the asthma management process, from prevention, to diagnosis, to treatment. Subsequently, the three mother-guidelines were searched for evidence to sustain the replies to the clinical questions and their corresponding recommendations. During the next stage each clinical question and its reply was analysed against the background of the Mexican reality, and a final set of suggestions and recommendations was drawn by the core-group. These were then submitted to the opinion of the broad GDG. Delphi rounds were employed to obtain the opinion and corrections of all members of the broad GDG. During the last face-to-face meeting final details of the recommendations were discussed and corrected, and the GL text was integrated. See Box 1 for the different steps in the development process.
Steps in the development of Guía Mexicana del Asma (GUIMA) 2017.
- I.
Selection of the guideline (GL) development group:
- i.
Executive Committee for Administration (JS), Content (DLL) and Financial support (JCV)
- ii.
Core group for GL development (4 pulmonologists and 4 allergists) [coordinating DLL and MCCS]
- iii.
Three methodologists, involved in all stages of development
- iv.
National Medical Societies, most related to the care of patients with asthma, giving autonomy to their Presidents to assign 3–5 members as collaborators for the guideline.
- i.
- II.
Elaboration of the document containing the objectives and the scope of the GL (SCOPE document)
- III.
Literature search for worldwide available asthma guidelines
- IV.
Evaluation of the quality of the GLs with AGREE II (2 evaluators/GL), and their adaptability to Mexican reality
- V.
According to IV, selection of the three best GLs as ‘Mother Guidelines’
- VI.
Development of flow-diagram of the different phases of asthma management, from diagnosis, to treatment, including crisis
- VII.
Formulation of key clinical questions for each asthma management step, using the PICO method
- VIII.
Answering of key clinical questions according to scientific evidence in Mother Guides, assigning level of evidence and recommendation
- IX.
With the complete Guideline Development Group fine-tuning of text of recommendations, using a Delphi process of several blocks and rounds
- X.
Integration of guideline text, based on the evidence set of key clinical questions
- XI.
Review final GL text with the complete Guideline Development Group, external reviewers (experts, legislators)
- XII.
Completion of the final GL document
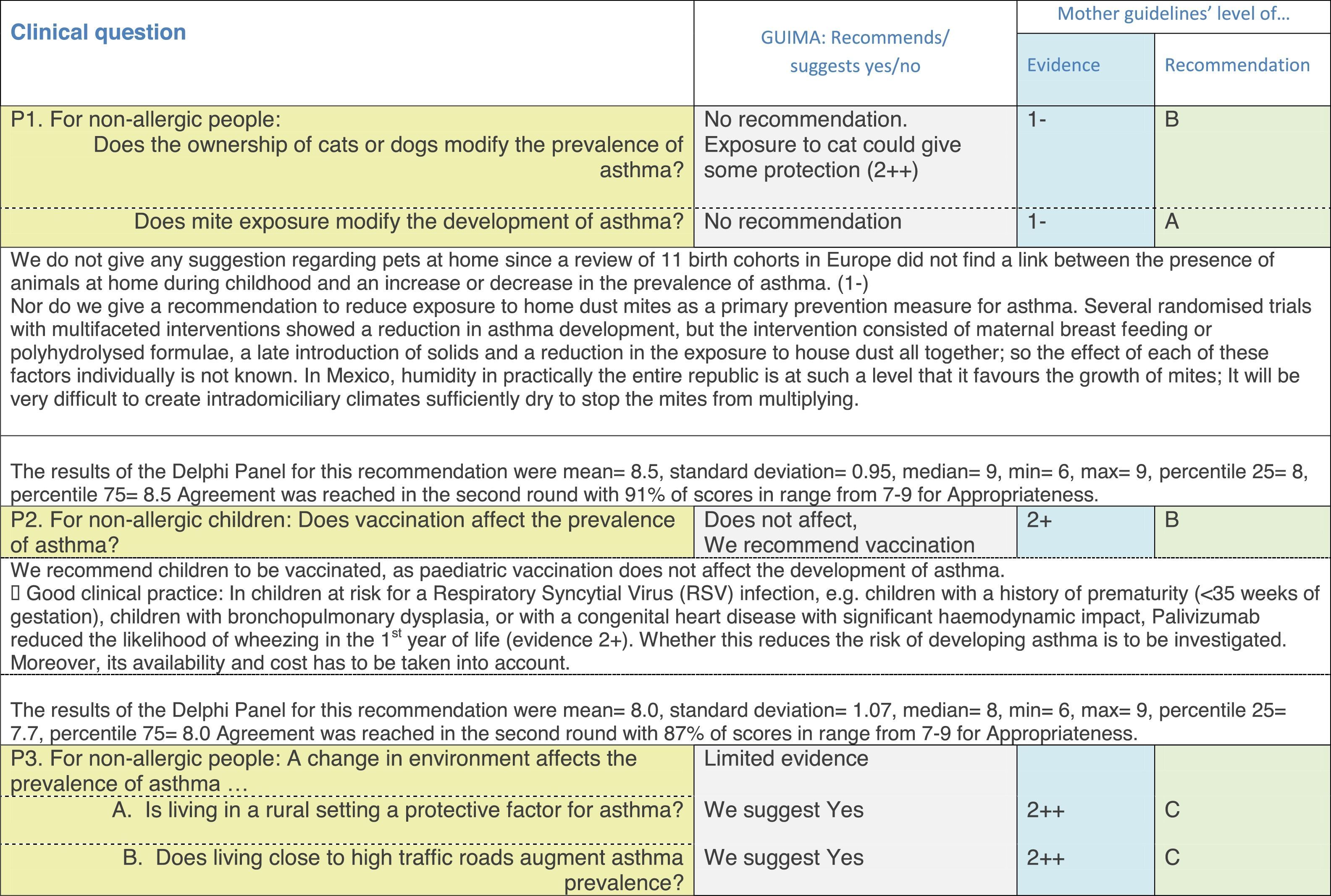
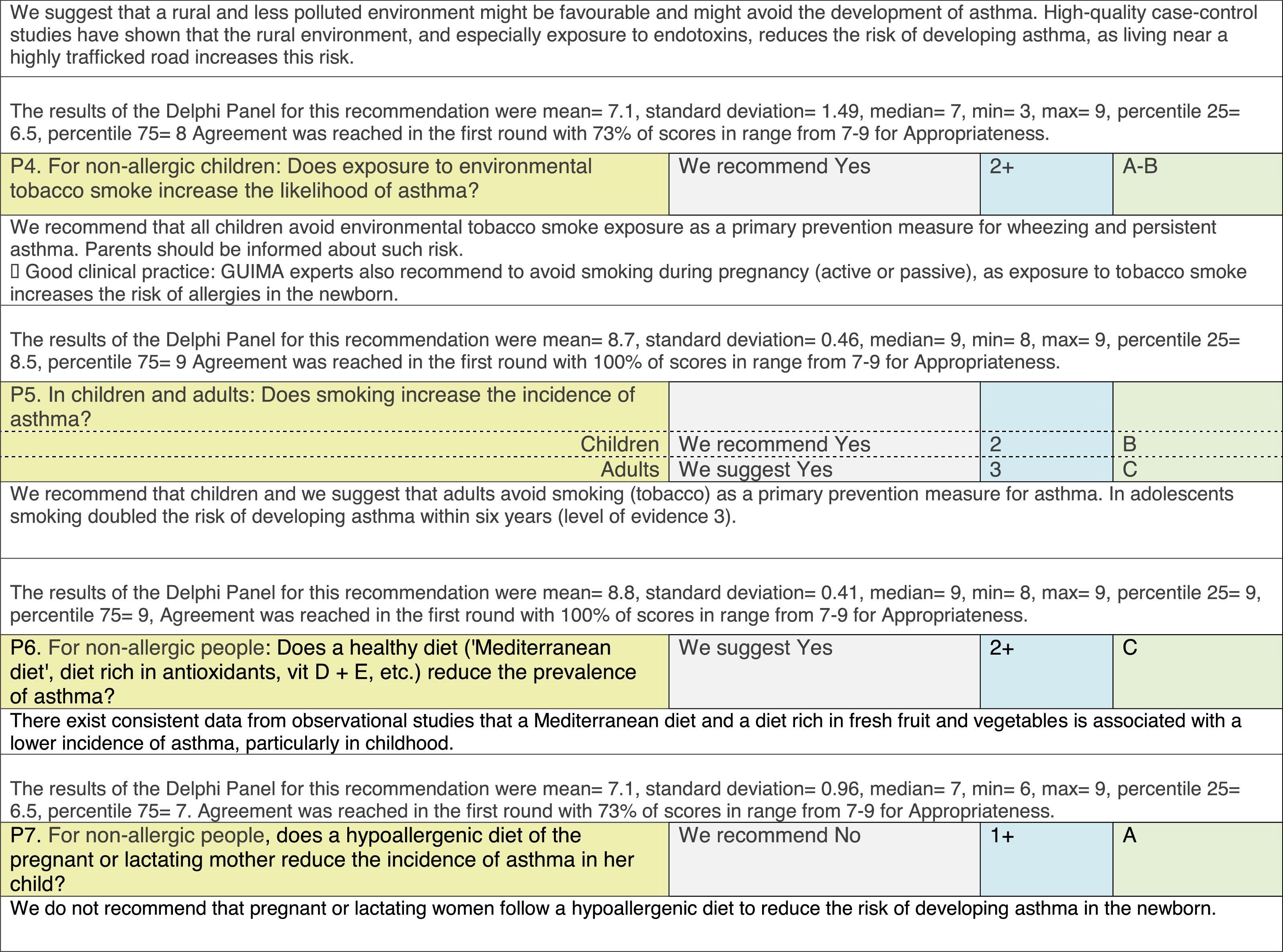
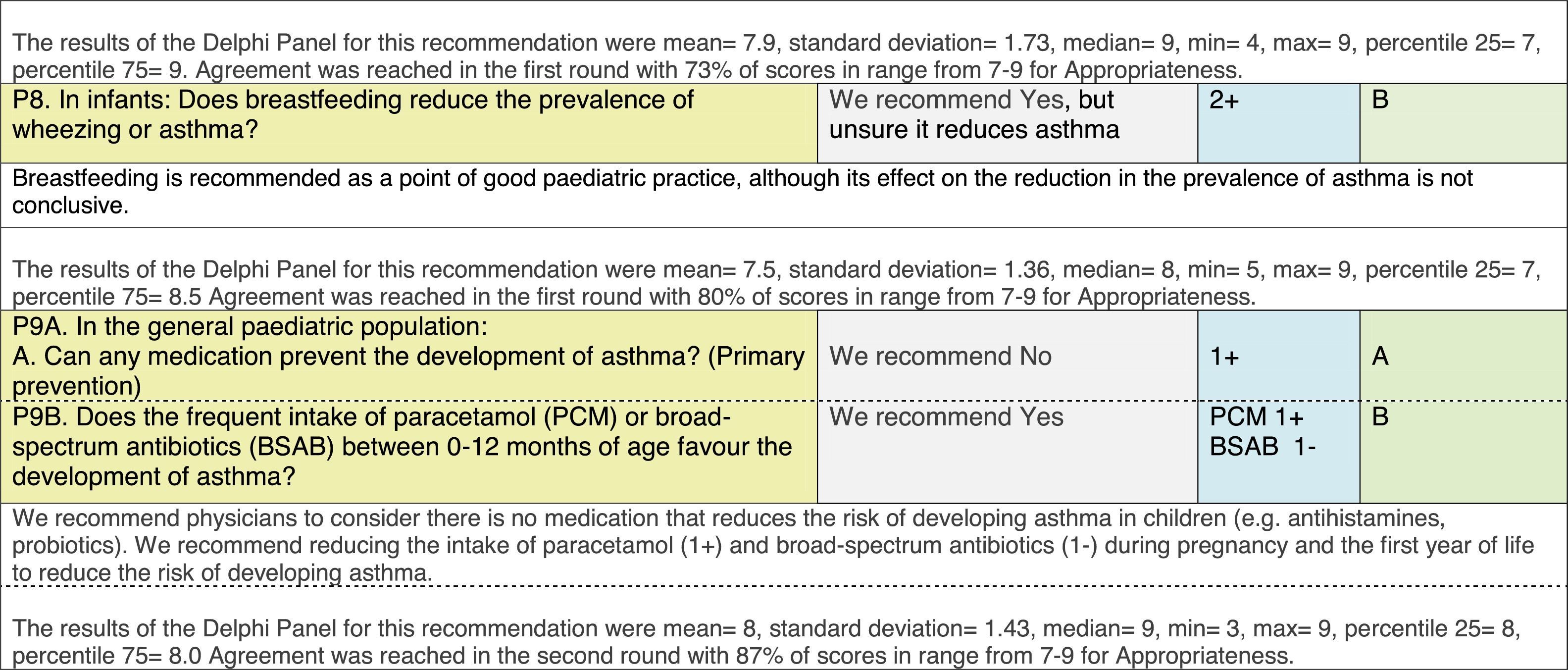
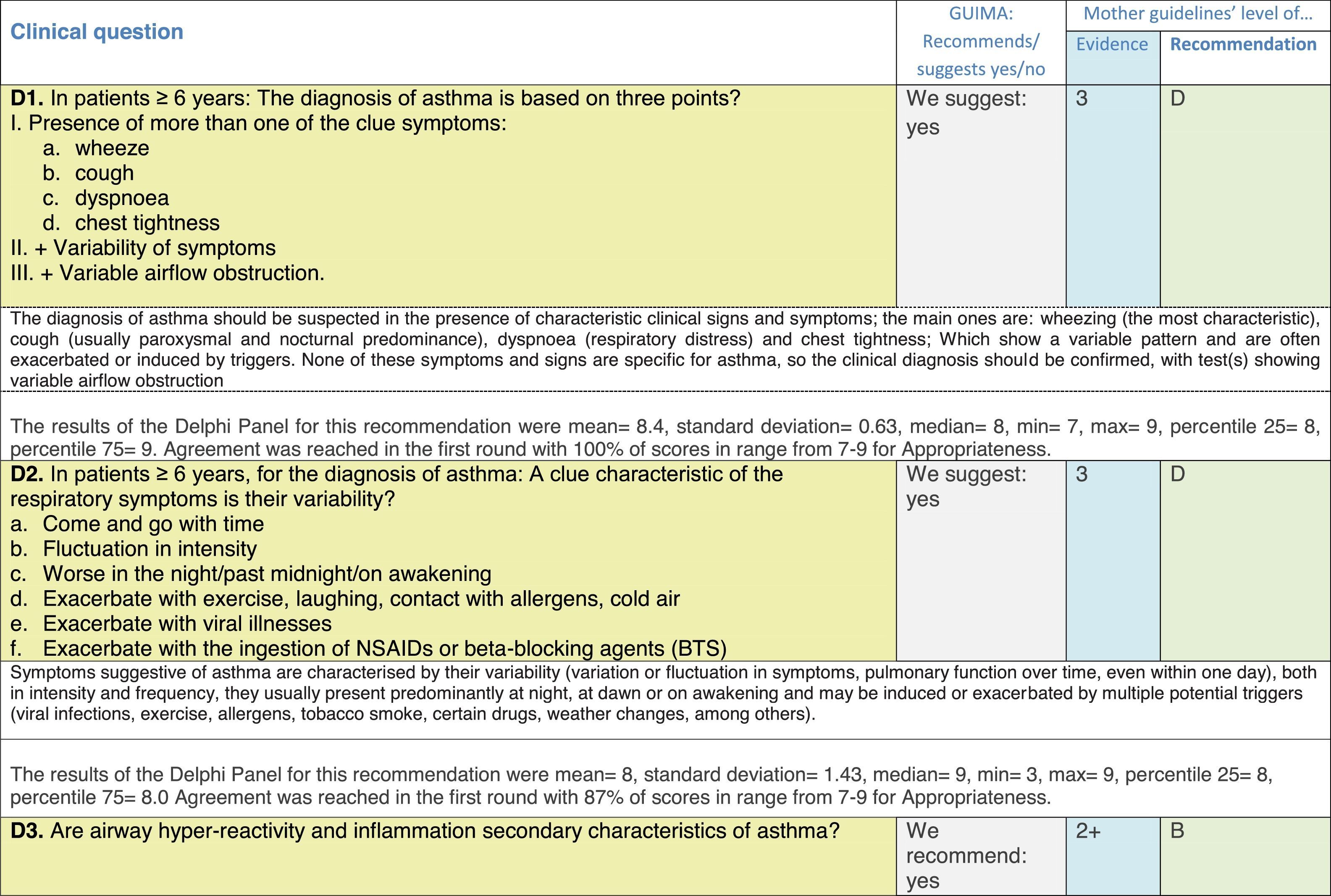
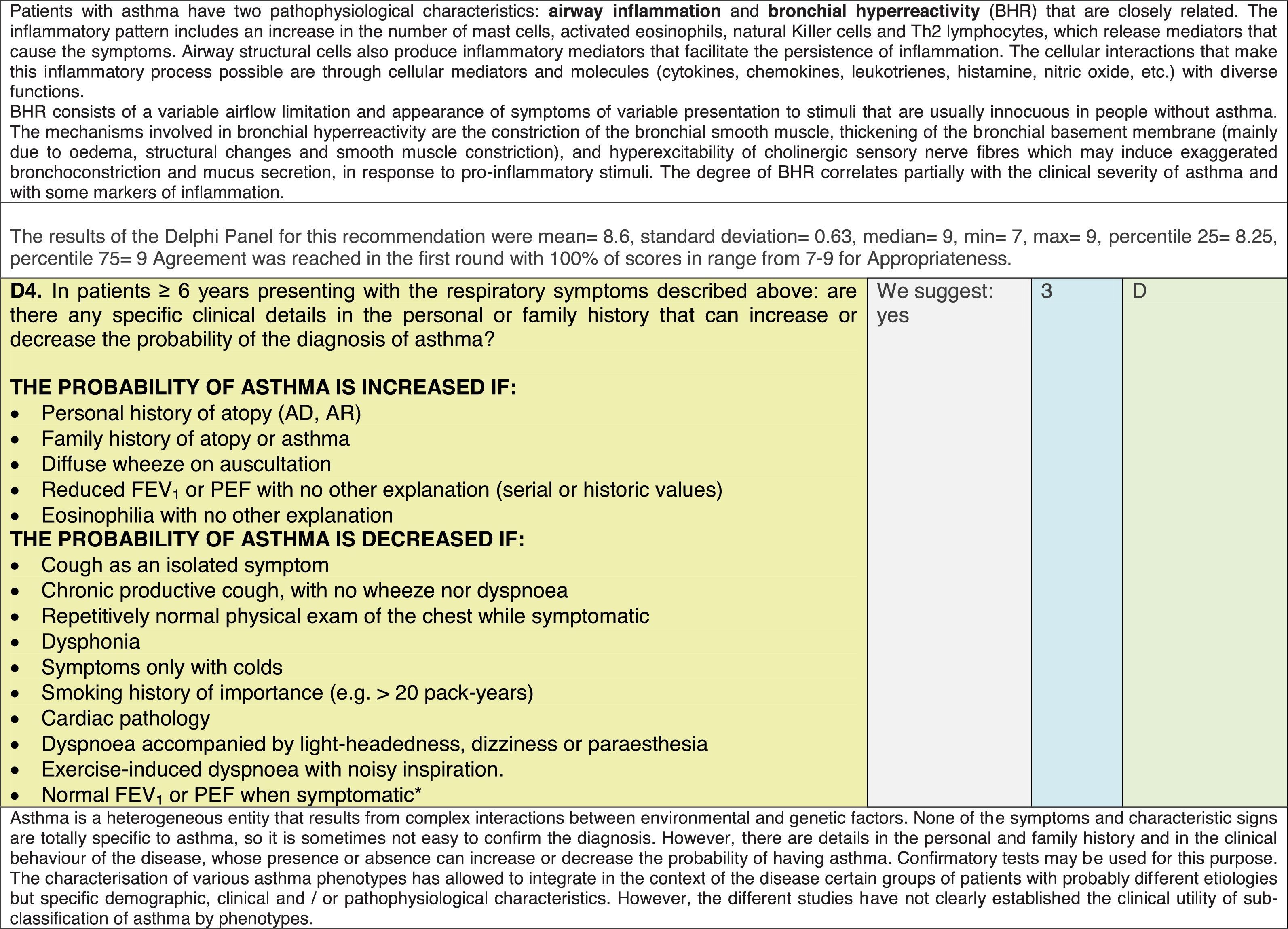
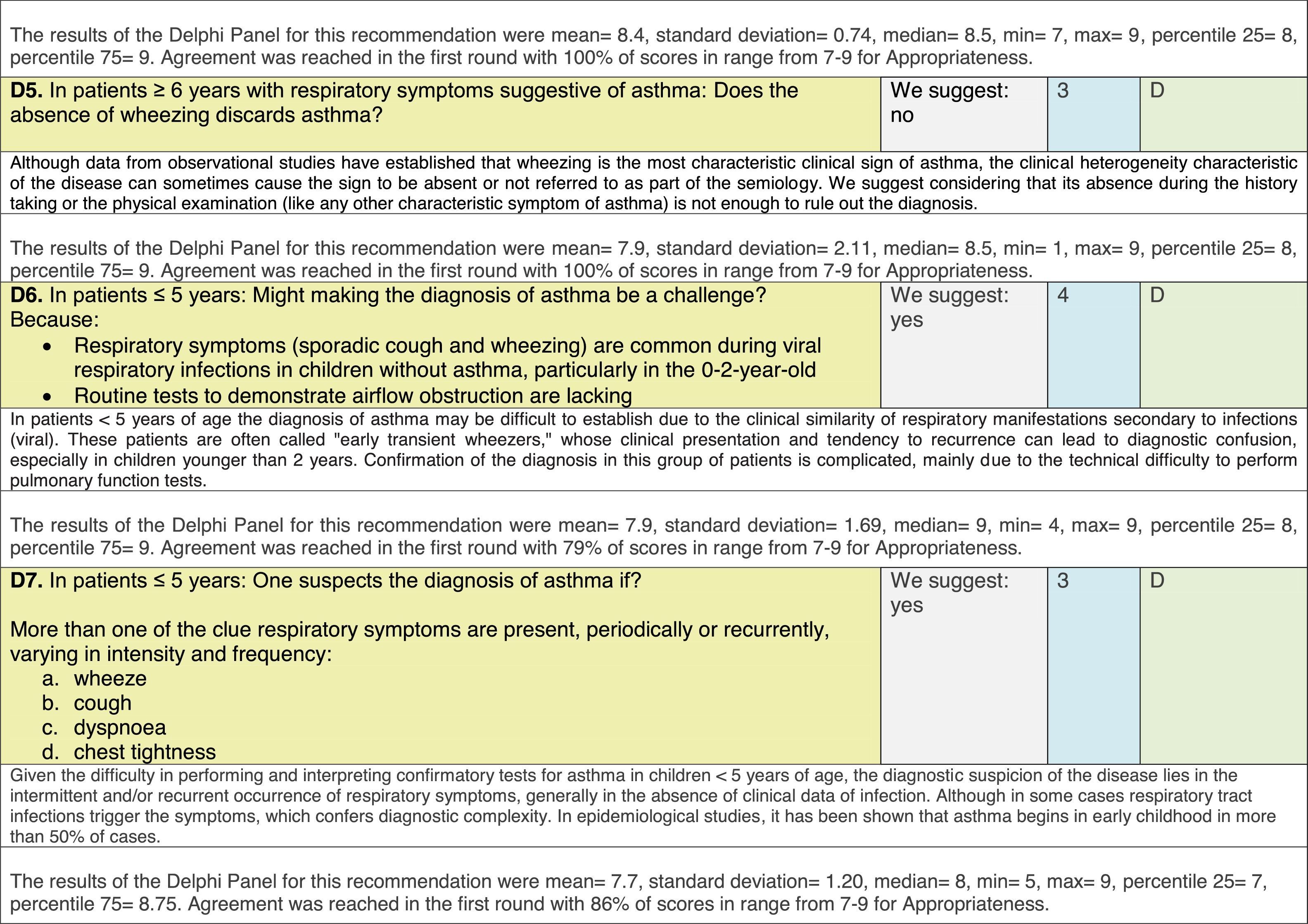
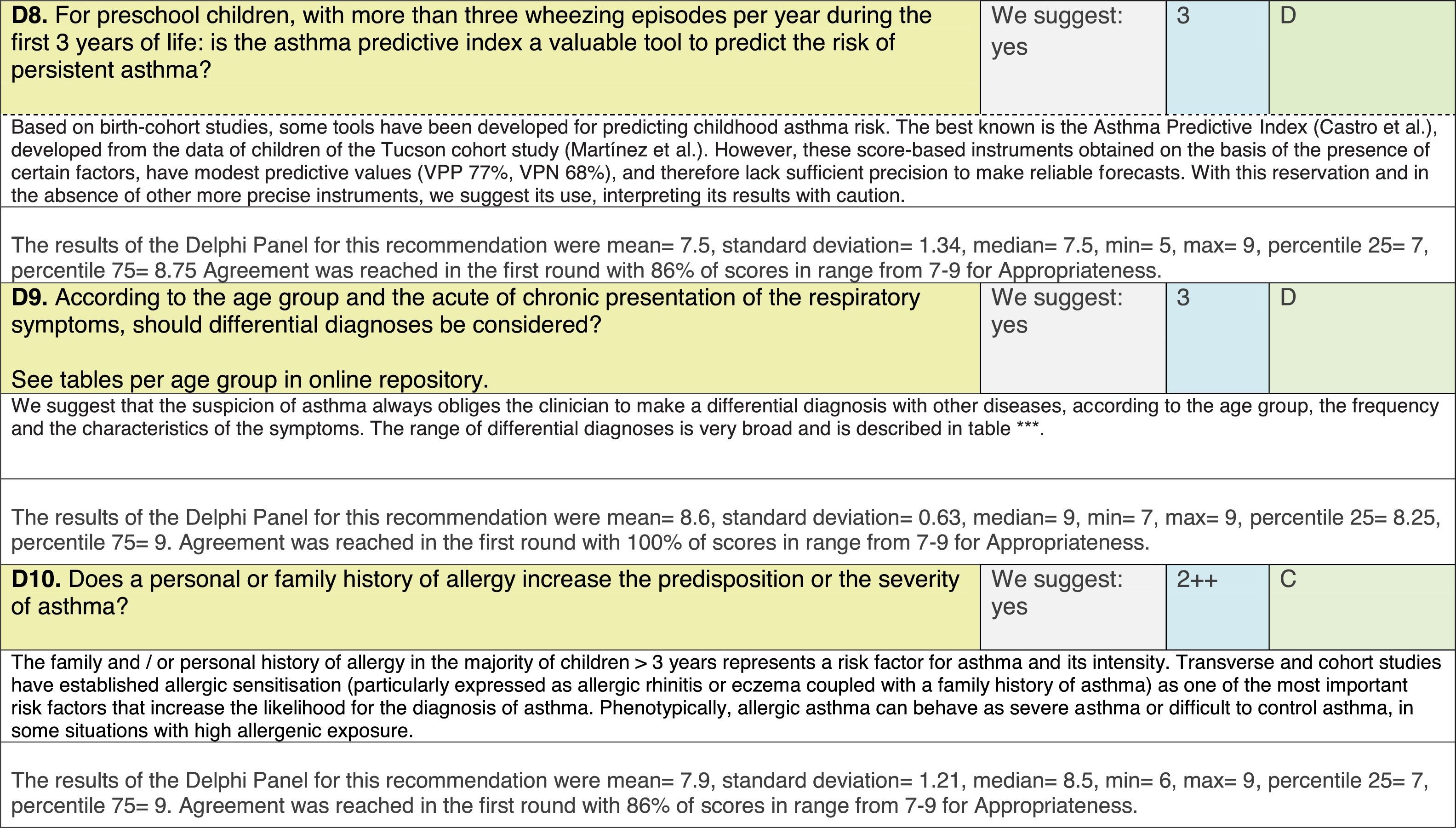
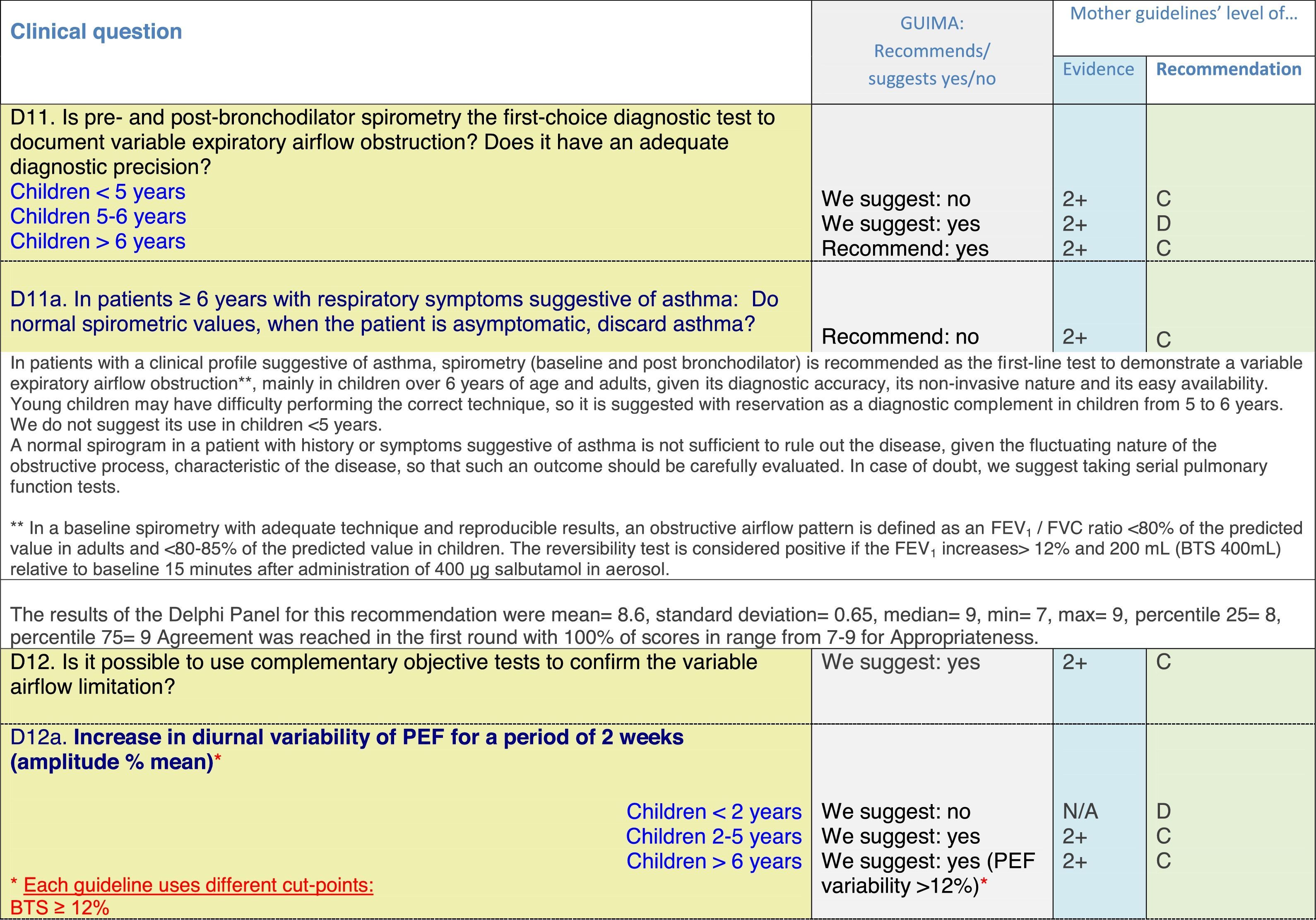
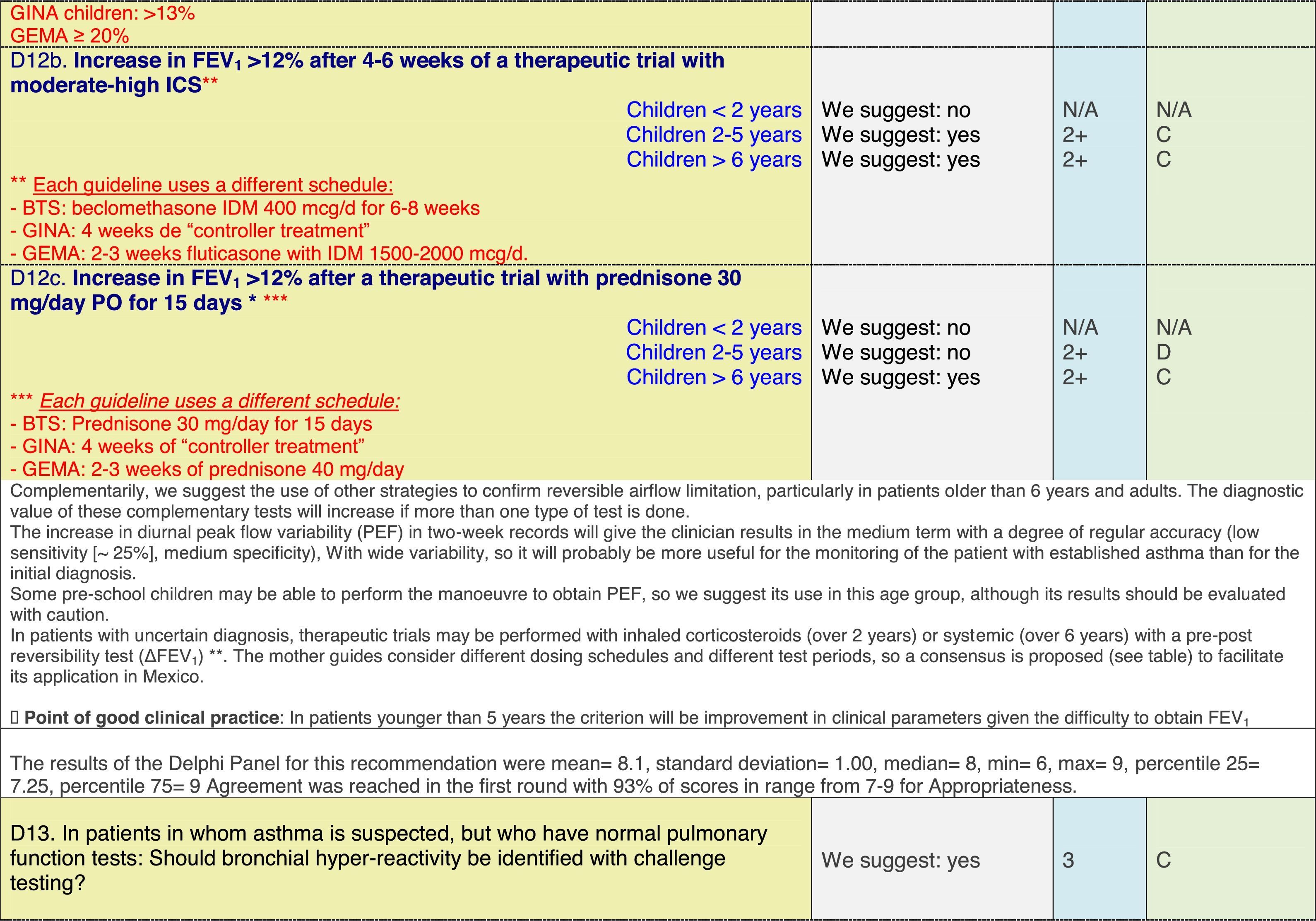
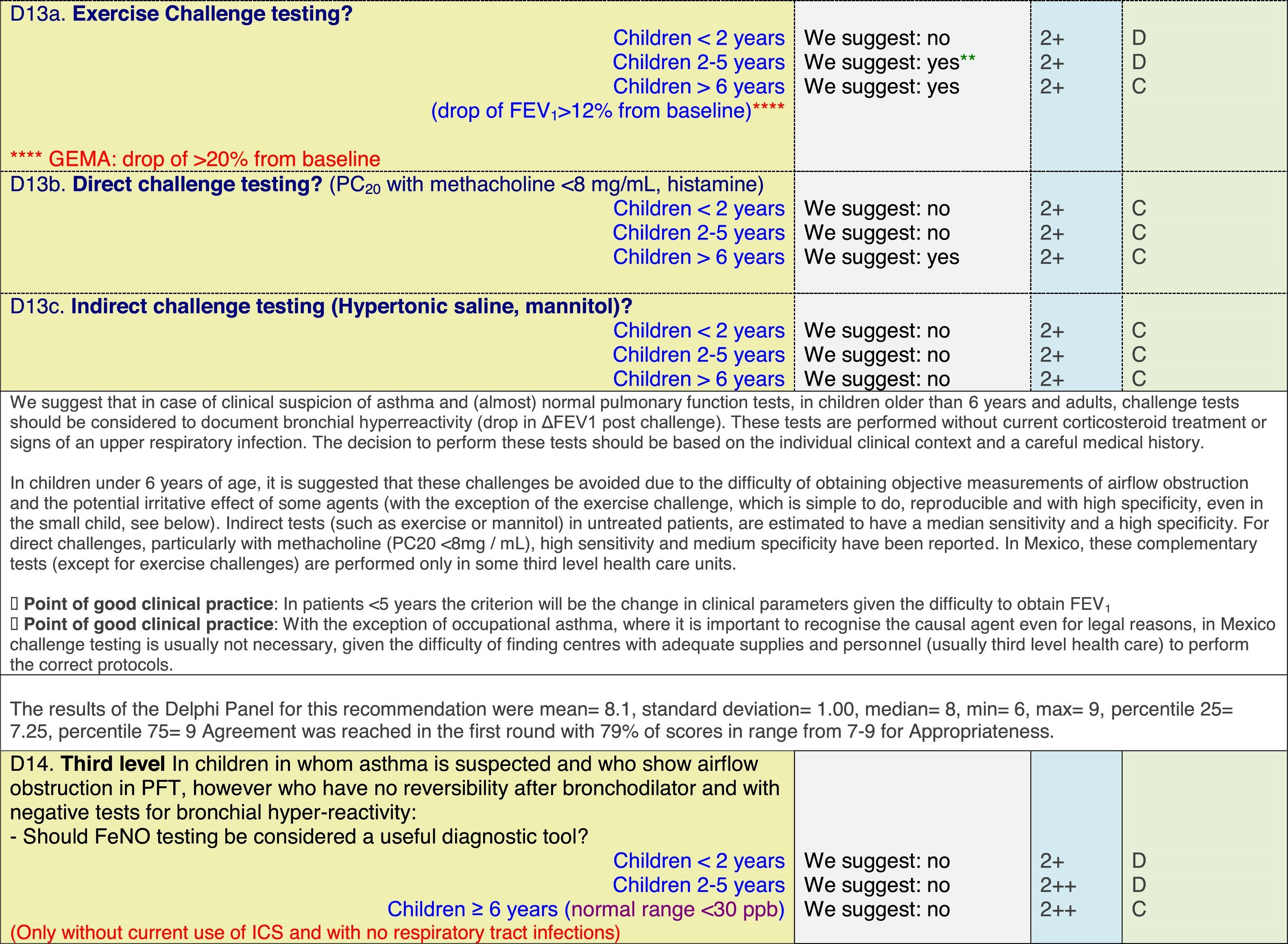
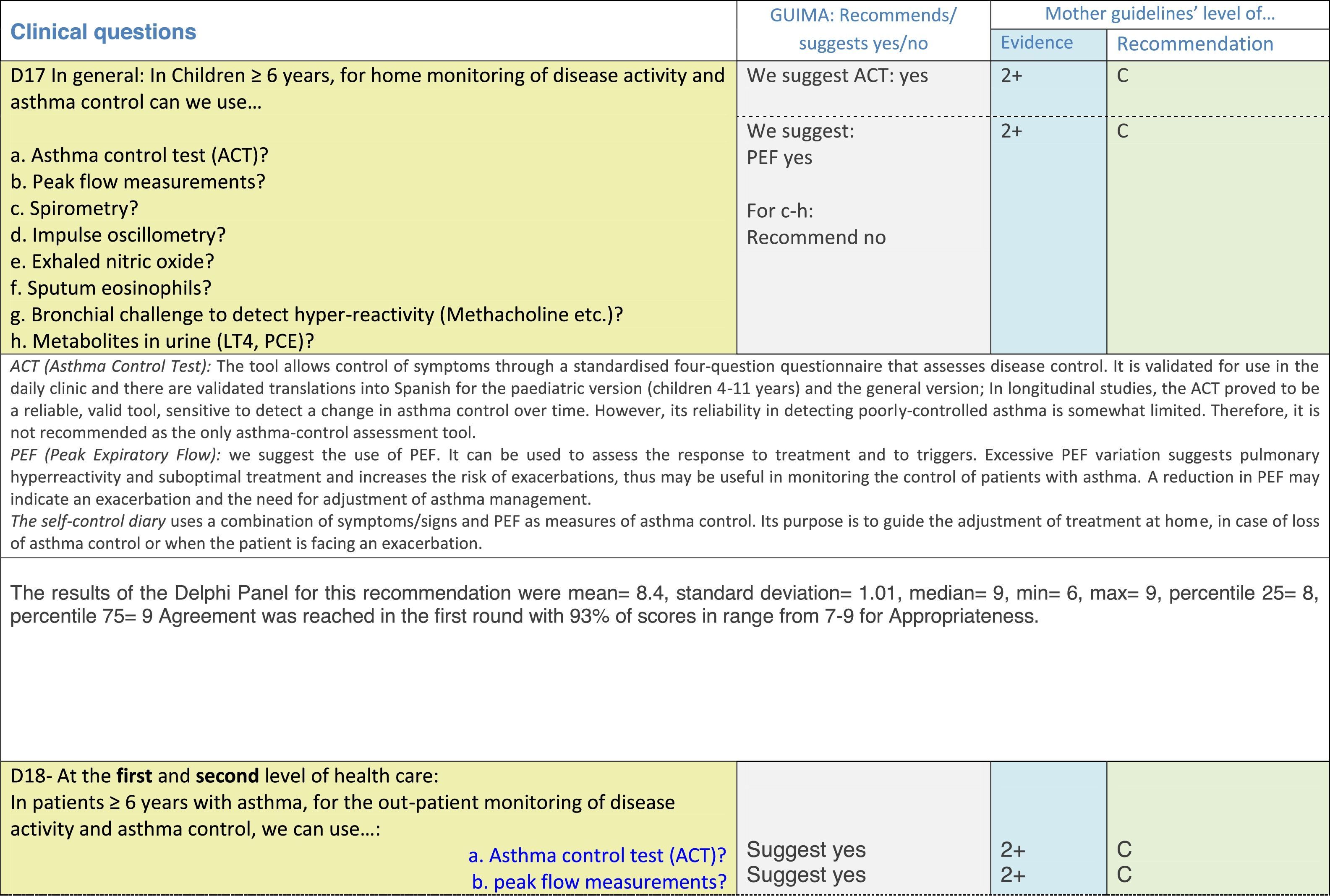
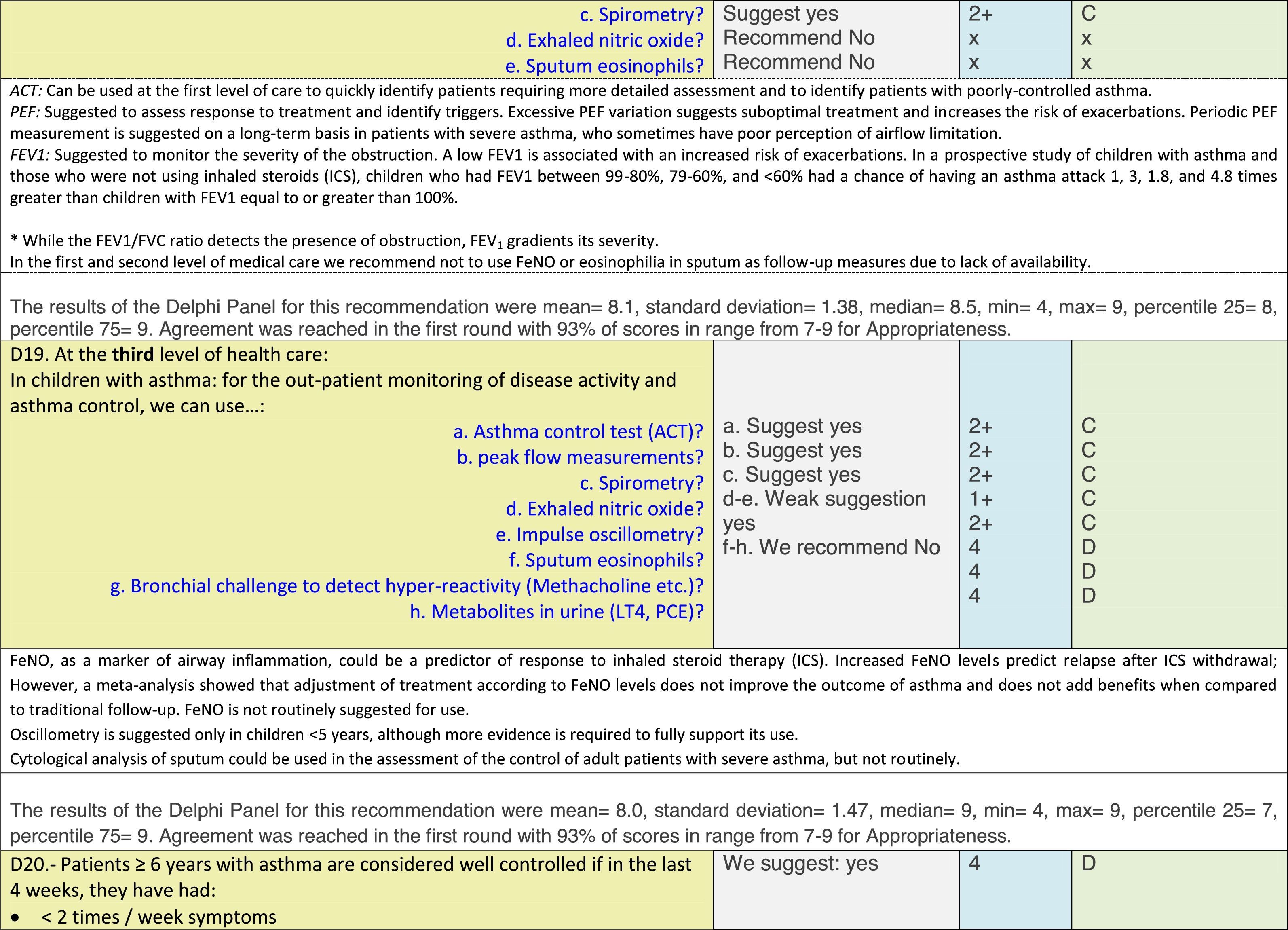
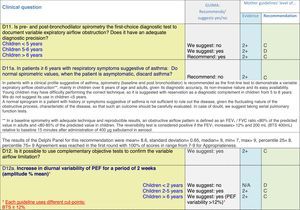
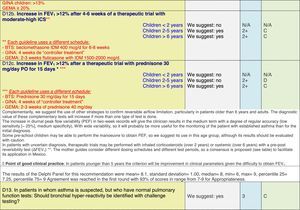
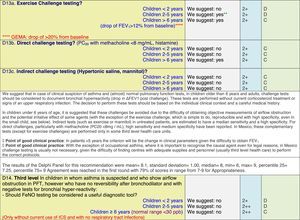
The results of step IX, Box 1, related to the clinical questions on asthma prevention and diagnosis in children are presented here, with their level of evidence and recommendation, fused from the three ‘mother-guidelines’. A brief clarification in the context of the Mexican reality accompanies the recommendation per question. To ‘recommend’ or to ‘suggest’ a certain action we used the Grading of Recommendation, Assessment, Development and Evaluation (GRADE) approach,11 in which a soft recommendation (suggestion) or strong recommendation is given, depending on the quality of the evidence, the cost and safety of a certain action or medication and the patient's preference. In the evidence column, the combined evidence from the three mother guidelines is depicted. In case of discordance between the mother guidelines, the original papers on which the evidence levels are based were reviewed, to come to a final level of evidence evaluation. In the final column, the joint level of recommendation is stated. For the separate evidence and recommendation analysis per guideline (before fusion), see the online repository. After each clinical question and the proposed reply for Mexico, the results of the Delphi rounds are stated, reflecting the level of acceptability and concordance among the GDG members with regards to the recommendation for Mexico.
ResultsTable 1 through Table 4 show the analysis of the recommendations and suggestions, as present in the mother guidelines and set in the context of Mexico. The results of the Delphi process can be found at the end of each question, including the level of agreement among the members of the GDG and the number of rounds that were necessary to come to agreement.
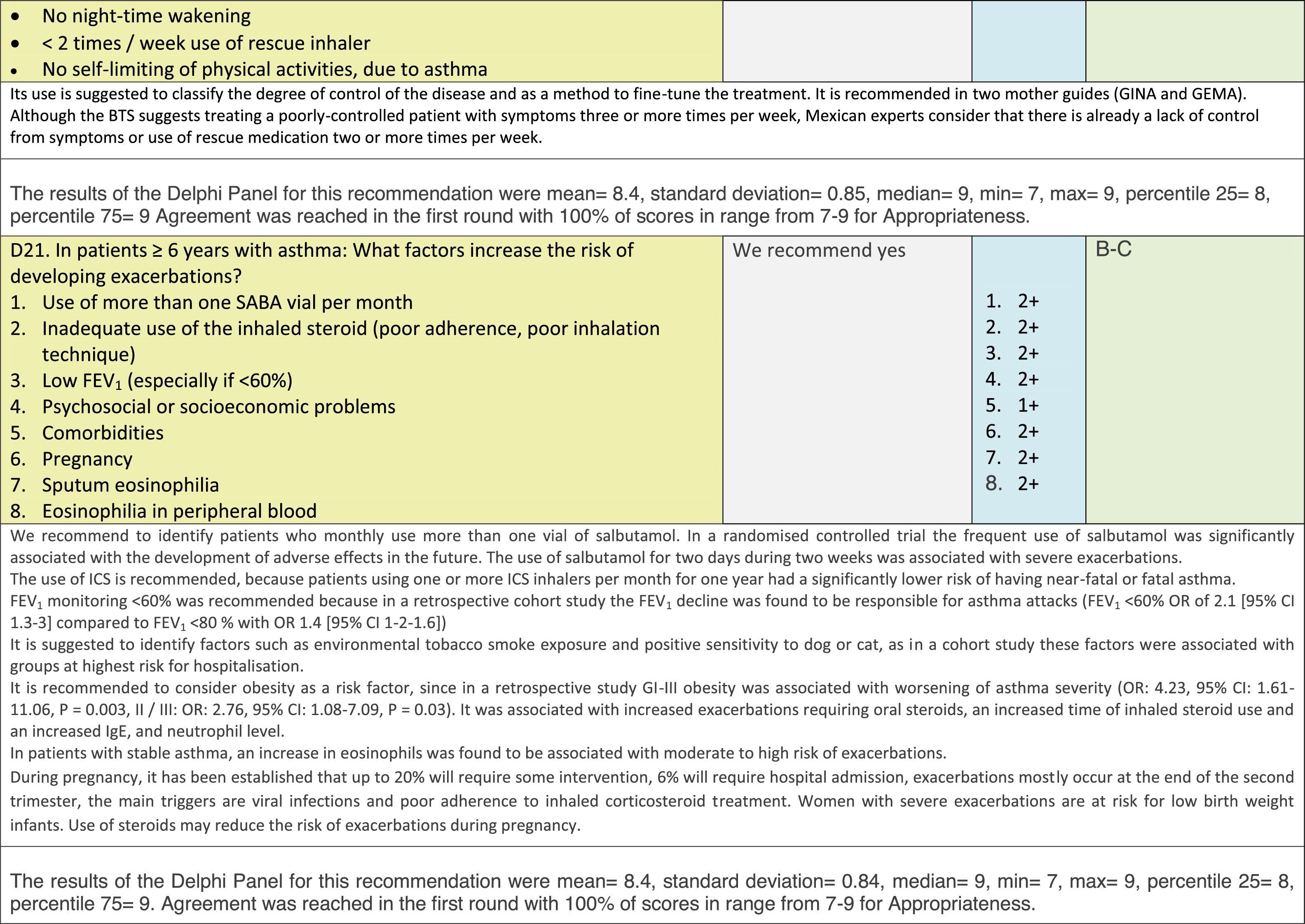
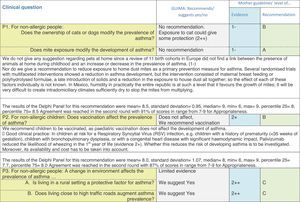
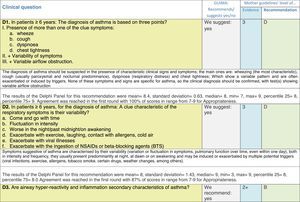
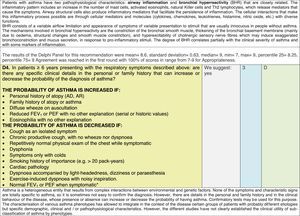
Issues related to the prevention of asthma are analysed in Table 1. The first part of the asthma diagnosis process, including the steps leading to suspecting the diagnosis of asthma is analysed in Table 2. The whole analysis of the diagnostic part starts off with the note that asthma is a heterogeneous entity, with a mostly clinical diagnosis. Thus, no strong evidence-based recommendations, related to its diagnosis, can be made. Table 3 explores the different tests that can be used to confirm the diagnosis of asthma. Finally, Table 4 focuses on establishing asthma severity and levels of asthma control.
Clinical questions on tests that confirm the diagnosis of asthma,a level of evidence and recommendation.
Analysing the results of the fusing of evidence from our three mother guidelines, it can be concluded that in the majority of the clinical questions the replies from the three guidelines are similar or complementary. For asthma prevention, there are only two firm recommendations: (1) avoidance of exposure to tobacco smoke, and (2) avoidance of the frequent intake of paracetamol (PCM) or broad-spectrum antibiotics (BSAB) between 0 and 12 months of age. Furthermore, it can be suggested to avoid living close to highly transited roads, in favour of the rural environment. Paediatric vaccination should be carried out with no restriction, just as for the population at large; pets at home should be allowed and there should be no restrictions to the mother's diet. To date, no medication exists that can avoid asthma development. For the diagnosis of asthma, the level of evidence is low, and in some cases absent, leaving only expert opinion to make suggestions. Generally, the presence of at least two of the four clue symptoms of asthma: wheeze, cough, dyspnoea or chest tightness, varying over time in presence and severity should raise the firm suspicion of asthma. The confirmation of asthma should be sought with the second line tests that demonstrate airflow obstruction and its variability over time: pre-post bronchodilator spirometry, serial peak flow measurements or a therapeutic trial. The mother guidelines differ somewhat in the exact cut-off points used in these tests.
In small children, the modified asthma predictive index could be used to try to evaluate the risk of developing asthma in children 0–3 years old with a history of four wheezing episodes. A personal or family background of atopy increases the risk of developing asthma and its severity.
Challenge testing could be of help in patients with the suspicion of asthma, who fail to demonstrate airflow obstruction or reversibility. These tests are normally reserved for third level health care, with the exception of the exercise challenge test that could be performed at any level, as long as the patients are closely observed during the testing. It is suggested not to use the fraction of exhaled nitric oxide (FeNO) test to confirm asthma, but as a measure of airway inflammation it can be of use for the follow-up in eosinophilic asthma. On the contrary, the demonstration of specific immunoglobulin E to a certain allergen is recommended at any age, when asthma symptoms flare after allergen exposure.
For the follow-up of the patients with asthma, again the level of evidence is mostly 2+ (low evidence), with a level of recommendation C. The asthma control test (ACT) is suggested by the mother guidelines, as are serial peak flow measurements and simple spirometry. The mother guidelines agree on a list of several factors that might indicate the risk of developing an asthma exacerbation (see Table 4, last rows).
ConclusionWe have shown how to take advantage of well-designed globally available guidelines for the development of a national guideline. It is possible to take the evidence from the best existing guidelines, analyse it in detail, fuse it and adjust it, according to local knowledge and reality, to come to a national recommendation for certain medical actions. We have applied this schedule for the development of the Mexican Asthma Guidelines and presented the section of prevention and asthma diagnosis in children in this article, which shall be followed by a similar analysis of asthma treatment in children.
Ethical disclosuresConfidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Protection of human subjects and animals in researchThe authors declare that no experiments were performed on humans or animals for this investigation.
FundingThe Guía Mexicana del Asma (GUIMA) 2017 project received unrestricted grants from Astrazeneca, Boehringer Ingelheim, Chiesi, GSK, Novartis and TEVA.
Conflict of interestThe following co-authors reported receiving honoraria as a speaker, support for congress attendance and/or grants of the indicated companies: DLL: AstraZeneca, Boehringer Ingelheim, MSD, Novartis, Grunenthal, Meda, Sanofi, UCB, Pfizer, TEVA, GSK, Amstrong, Siegfried, DBV technologies. MDCCS: Takeda. MAJC: Janssen, MSD. SJRT: Boehringer Ingelheim. BDRN: Sanofi, MSD, Grunenthal. JALP: Mead & Johnson Nutricionales, Meda. JAOM: UCB, AstraZeneca, Sanofi. JVR: A2DAHT. NRP: MSD, AstraZeneca, Boehringer Ingelheim. CGG: AstraZeneca. DAMH: Senosiain. HHRG: Abbvie, Novartis, Roche. HLM: Aerosol Medical Systems. JGV: Novartis, Sanofi. JLMR: AstraZeneca.
We would like to thank Gustave Giraldo, Angélica Moctezuma and Mario Rodriguez for their support with logistics. We would like to thank the staff of the Sociedad Mexicana de Neumología y Cirugía de Tórax for their unconditional support with logistics and administrative issues. We would also like to thank Astrazeneca, GSK, Boehringer-Ingelheim, Chiesi, MEDA, Novartis and TEVA for unrestricted grants.