Eczema is one of the most common inflammatory diseases, often constituting a lifelong burden for afflicted individuals. The complex interaction of host genetic and multiple environmental factors contribute to its pathogenesis. A relationship between maladjustment of gut microbiota and eczema has been brought into the light of day in most previous studies. In eczema preclinical models, specific intestinal microbial species have been demonstrated to prohibit or dwindle immune responsiveness, indicating that these strains among commensal gut bacteria may exert either a morbific or phylactic function in eczema progression. As such, oral probiotics can serve as a medicinal approach for eczema therapy. Given that relative scientific work is still at the early stage, only limited data are available in the field. New sequencing techniques have been fortunately performed to gain access to an extended research on the relationship between gut bacterial flora and human diseases. In the current review, we identified the role of intestinal microbiota in the development of eczema and how specific bacterial strains adjust the immune responsiveness in the midst of disease progression. Probiotics as an applicable treatment for eczema were evaluated in other threads as well.
Eczema, also known as atopic dermatitis, is a common childhood condition characterised by the inflammation of skin with intense itching.1 The prevalence of eczema has been increasing worldwide during the past decades, particularly in industrialised nations and amongst children.2–4 Even though research studies on genetic predisposition to eczema have implicated genes (e.g. IL4, IL4R, IL13, CMA1, SPINK5, FLG, IL-6),5–12 the aetiology of systemic inflammation still requires further investigation. The gut is a crucial immune organ besides its function in metabolism and in the body it includes the biggest lymphoid tissue mass.13 The gut is a habitat to vast and various species of microbes.13,14 Crucial signals derived from the gut microbiota contribute to the development of host immunity. Hence, Gut microbes are very important to maintain human health and disease. Recent studies in experimental subjects have demonstrated that maladjustment of the intestinal microbiota is related to the development of eczema.15,16 Changes in terms of the abundance of certain intestinal microbial species have been revealed to prohibit or dwindle immune responsiveness in eczema experimental subjects, they may be biomarkers in eczema prevention and therapy. In spite of being a relatively novel area of research, evidence hitherto indicates that the intestinal microbiota may serve as fecund targets for prevention or control of eczema which is primarily characterised by innate and adaptive immune dysbiosis. In the current study, we reviewed recent series to further investigate changes in microbiota composition which trigger eczema disease, and the efficacy of probiotics/prebiotic in the therapy for eczema is also assessed based on the previous studies.
Eczema pathogenesis and the role of gut microbiota within itEczema pathogenesisEczema is a chronic form of childhood disorder that is characterised by remitting and relapsing cutaneous symptoms. These symptoms include itching and dryness, flaking, blistering, oozing, and bleeding.17 Eczema is often the first manifestation of atopy in infants who will develop asthma or allergic rhinitis later in childhood.18,19 Different dendritic cells subtypes, such as Langerhans cells (LC), inflammatory dendritic epidermal cells (IDEC) and plasmacytoid dendritic cells (PDC), play a key role in eczema and impact on the mechanisms underlying eczema, such as the recruitment of inflammatory cells, T-cell priming, and cytokine and chemokine release. Lesional skin of eczema patients harbours significant numbers of LC; IDEC and PDC expressing the high-affinity receptor for immunoglobulin E (IgE).20–22 An enhanced T helper 2 (Th2) immune response, reflected by an increased frequency of allergen-specific T cells producing interleukin (IL)-4, -5 and-13, and a decrease in interferon (IFN)-γ-producing T-cells.23,24 IL-4 can be involved in IgE isotype switching, while IL-5 can attract eosinophils and prolong their survival, which may result in the peripheral blood eosinophilia and increased IgE serum levels in eczema patients.25 Moreover, there is a preferential apoptosis of circulating Th1 cells in eczema, which may also contribute to Th2 predominance in eczema patients.26 Interestingly, eczema patients have significantly increased numbers of circulating regulatory T cells that exhibit normal immunosuppressive activities in vitro.27 Studies on genetic predisposition to eczema have implicated eczema-related genes such as (e.g. IL4, IL4R, IL13, CMA1, SPINK5, FLG, IL-6),5–12 environmental factors have also been shown to contribute to the disease pathogenesis. There are also other possible mechanisms that aberrant barrier functions in gut mucosa lead to greater antigen transfer across the mucosal barrier and the routes of transport are altered, thereby evoking aberrant immune responses and release of pro-inflammatory cytokines with further impairment of the barrier functions.28 Such increased inflammation would lead to further increases in intestinal permeability and in a vicious circle of increasing allergenic responses, and a more permanent dysregulation of the immune responses to ubiquitous antigens in genetically susceptible individuals.29 The alteration of gut microbiota has an important impact on the peripheral and central immune system. Many unclear mechanisms still exist.
The role of gut microbiota in eczemaRecently, it has been indicated that microbial triggers have been implicated in eczema.30 The vast majority of these studies suggested that subjects with eczema exhibit alterations in the relative abundance of “beneficial” and potentially “harmful” bacteria compared to healthy subjects (Table 1). There is convincing evidence from experimental subjects suggesting that kind of factors (e.g., diet, level of physical activity, during pregnancy, and with the use of broad-spectrum antibiotics) are related to eczema disease via affecting gut microbiota. The existence of a link between eczema and the gut microbiota was indicated based on studies in patients with eczema. Infants with eczema harboured significantly lower gut microbial diversity when compared to healthy controls, suggesting that alteration of the entire intestinal microbiota and the lack of exposure to certain bacterial targets may be contributing factors that amounted to their diseased state.31,32 In keeping with the observation, by comparing with healthy subjects, gut microbial diversity significantly decreased in infants with IgE-associated eczema, the diversity of the bacterial phylum Bacteroidetes and phylum Proteobacteria also significantly reduced, the level of the phylum Proteobacteria significantly decreased.33Proteobacteria comprises gram-negative bacteria, typically with endotoxin lipopolysaccharides (LPS) incorporated into the cell wall. Endotoxin can induce a TH1 response through the innate immune system by enhancing IL-12 production from monocytes and dendritic cells,34 and low exposure to endotoxin has been associated with an increased risk of atopic eczema.35 In addition, a strong endotoxin exposure might down regulate atopy-promoting Th2 responses.33 Similarly, Nylund et al.,36 also found that infants with eczema appeared to have a significant decrease in the abundance of Bacteroidetes compared to healthy infants. Therefore, these increasing studies suggest that changes in the composition of gut microbiota play a significant role in induction and furthering the progression of eczema. The abundance of Ruminococcaceae was significantly lower at one week of age in infants with IgE-associated eczema than controls.37 Meanwhile, the abundance of Ruminococcus was significantly negatively associated with TLR2-induced IL-6 and TNF-α. The abundance of the phylum Proteobacteria and the family Enterobacteriaceae significantly decreased in infants with IgE-associated eczema compared to controls. The abundance of Proteobacteria was significantly inversely related with TLR4-induced TNF-α. The abundance of Enterobacteriaceae was significantly negatively associated with TLR4-induced TNF-α and IL-6. At one year, α-diversity of Actinobacteria was significantly lower in infants with IgE-associated eczema compared with controls. Ruminococcaceae belonging to Firmicute have been associated with the maintenance of gut health. There is emerging interest in the role of Ruminococcus colonisation in infancy.38 A possible health benefit is the production of ruminococcins, such as ruminococcin A, which is a bacteriocin that can inhibit the development of Clostridium species.39 Thus, reduced abundance of potentially immunomodulatory gut bacteria is associated with exaggerated inflammatory cytokine responses to TLR-ligands and subsequent development of IgE-associated eczema. Abrahamsson et al.33 reported the diversity of the bacterial genus Bacteroides to be significantly reduced. Bacteroides species have also been demonstrated to have anti-inflammatory properties. Bacteroides can break down complex plant polysaccharides40 and their abundance has been associated with increased short-chain fatty acid concentrations in the infant gut after introduction of the first solid foods.41 Furthermore, B. fragilis polysaccharide has been shown in a mice model to direct the cellular and physical maturation of the developing immune system via its ability to direct the development of CD4+ T cells, thus inducing the differentiation of Th1 lineage and correction of the Th1/Th2 imbalance.42 All in all, the Bacteroides have significance in the development and maintenance of gut and balanced mucosal immunity. In accordance with the observation, Watanabe et al.,43demonstrated eczematous subjects had significantly lower counts of Bifidobacterium than healthy subjects, and the frequency of occurrence of Staphylococcus was significantly higher in eczematous subjects than in healthy subjects. Bifidobacterium can stimulate the production of Th1-type cytokines and leads to Th1-dominant immunity.44 Therefore, the amount of Bifidobacterium in the intestine might be related to the onset of eczema in the host. Mah et al.,45 revealed toddlers suffering from eczema harboured significantly lower abundance of Bifidobacterium and Clostridium, but significantly higher counts of total lactic-acid-producing bacteria (LAB) and enterococci compared to controls. Similarly, another study shown children with eczema had increased abundance of the Clostridium clusters IV and XIVa,36 which are typically abundant in adults. Thus, children with eczema prematurely changed towards gut microbiota of adult-type. Gut microbiota of infant-type may enhance the normal mucosal barrier function by affecting the maturation of the gut epithelium and immune functions and reducing intestinal inflammation.46,47 Kirjavainen et al.48 testified infants with eczema in the highly sensitised group (HSG) had significantly greater numbers of lactobacilli/enterococci than those in the sensitised group (SG). Lactobacilli are probiotic bacteria, which can manipulate immune function, adhere to epithelial cells of the intestinal mucosa and colonise on the surface of gastrointestinal mucosa to form a bacterial membrane barrier to protect epithelial cells of the intestinal mucosa against injury from a variety of pathogenic microorganisms. Meanwhile, a number of other studies have also found that lactobacilli are associated with beneficial effects in the management of atopic eczema.49–51 A possible explanation is that allergic sensitisation promotes the growth of lactobacilli and/or enterococci by causing changes in the gut ecology. In addition, Some species of enterococci have virulence factors that can compromise the gut barrier and in theory could thereby affect atopic sensitisation.52 Hong et al.,17 found that Bifidobacterium was present at significantly higher abundance in non-eczema infants compared to those with eczema, while Enterococcus, Klebsiella and Shigella were present at significantly higher abundances in eczema infants during early stages of infancy. Bifidobacterium was less diverse in eczema infants than the non-eczema group. For example, at one month of age, the B. angulatum, the B. adolescentis, B. dentium, the B. catenulatum group, the B. bifidum group and the B. longum group were only detected in non-eczema infants. At three months of age, the B. dentium and the B. bifidum group were only detected in non-eczema infants. At 12 months of age, the relative abundances of the B. longum group was significantly higher in non-eczema infants than eczema infants. Recently, one study found Campylobacter was significantly more abundant in infants with eczema and non-allergic controls, while Roseburia was significantly less abundant in participants with eczema than in controls.53Campylobacter can disrupt the intestinal epithelial barrier, which allowed the translocation of non-invasive bacteria such as Escherichia coli54 and primed the intestine for inflammatory responses in susceptible infants. Roseburia is a butyrate-producing bacterium inhibiting histone deacetylase activity, leading to hyperacetylation of histones and thus suppression of nuclear factor-kappa B activation, and reinforcing the colonic defence barrier by enhancing the production of mucins and antimicrobial peptides, as well as the expression of tight junction proteins.55 Disorder of the intestinal microflora might play a role in the onset of eczema and the aggravation of eczema.
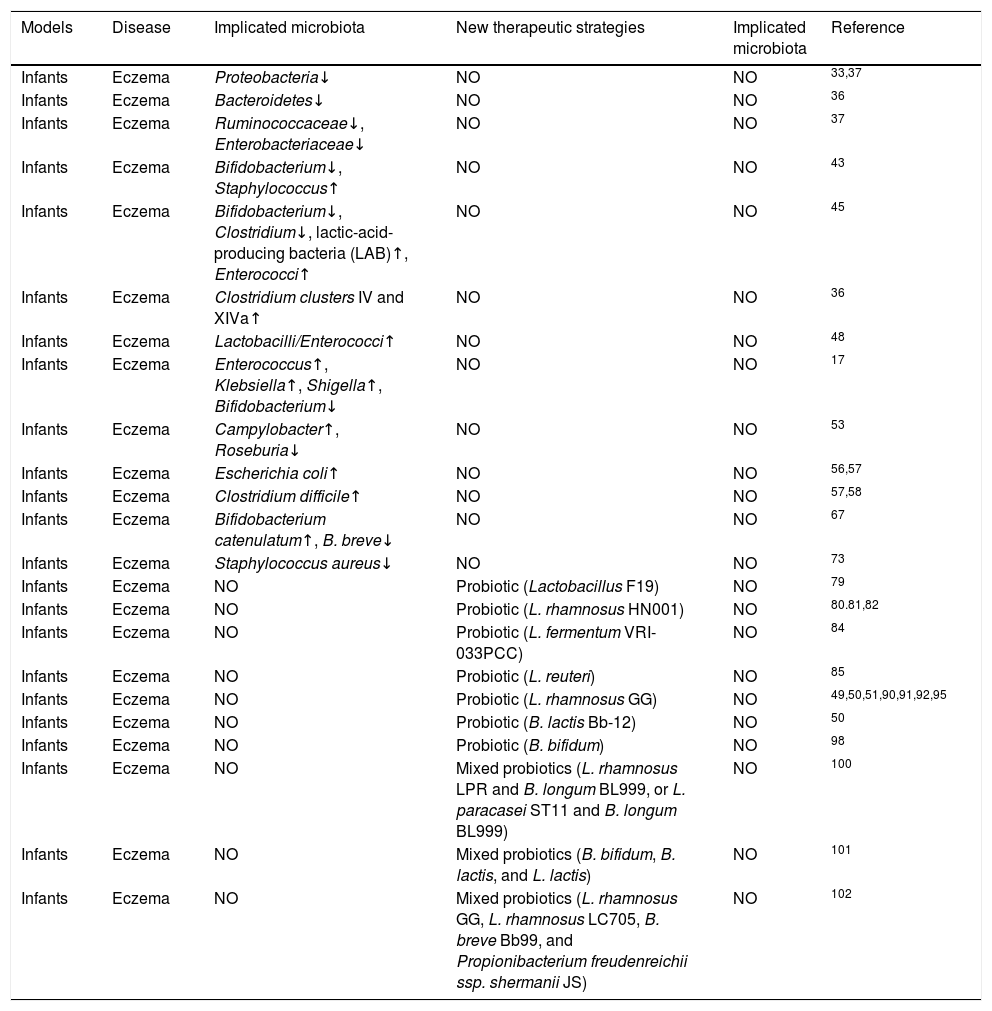
Changes in microbiota composition associated with eczema and potential therapeutic strategies.
| Models | Disease | Implicated microbiota | New therapeutic strategies | Implicated microbiota | Reference |
|---|---|---|---|---|---|
| Infants | Eczema | Proteobacteria↓ | NO | NO | 33,37 |
| Infants | Eczema | Bacteroidetes↓ | NO | NO | 36 |
| Infants | Eczema | Ruminococcaceae↓, Enterobacteriaceae↓ | NO | NO | 37 |
| Infants | Eczema | Bifidobacterium↓, Staphylococcus↑ | NO | NO | 43 |
| Infants | Eczema | Bifidobacterium↓, Clostridium↓, lactic-acid-producing bacteria (LAB)↑, Enterococci↑ | NO | NO | 45 |
| Infants | Eczema | Clostridium clusters IV and XIVa↑ | NO | NO | 36 |
| Infants | Eczema | Lactobacilli/Enterococci↑ | NO | NO | 48 |
| Infants | Eczema | Enterococcus↑, Klebsiella↑, Shigella↑, Bifidobacterium↓ | NO | NO | 17 |
| Infants | Eczema | Campylobacter↑, Roseburia↓ | NO | NO | 53 |
| Infants | Eczema | Escherichia coli↑ | NO | NO | 56,57 |
| Infants | Eczema | Clostridium difficile↑ | NO | NO | 57,58 |
| Infants | Eczema | Bifidobacterium catenulatum↑, B. breve↓ | NO | NO | 67 |
| Infants | Eczema | Staphylococcus aureus↓ | NO | NO | 73 |
| Infants | Eczema | NO | Probiotic (Lactobacillus F19) | NO | 79 |
| Infants | Eczema | NO | Probiotic (L. rhamnosus HN001) | NO | 80.81,82 |
| Infants | Eczema | NO | Probiotic (L. fermentum VRI-033PCC) | NO | 84 |
| Infants | Eczema | NO | Probiotic (L. reuteri) | NO | 85 |
| Infants | Eczema | NO | Probiotic (L. rhamnosus GG) | NO | 49,50,51,90,91,92,95 |
| Infants | Eczema | NO | Probiotic (B. lactis Bb-12) | NO | 50 |
| Infants | Eczema | NO | Probiotic (B. bifidum) | NO | 98 |
| Infants | Eczema | NO | Mixed probiotics (L. rhamnosus LPR and B. longum BL999, or L. paracasei ST11 and B. longum BL999) | NO | 100 |
| Infants | Eczema | NO | Mixed probiotics (B. bifidum, B. lactis, and L. lactis) | NO | 101 |
| Infants | Eczema | NO | Mixed probiotics (L. rhamnosus GG, L. rhamnosus LC705, B. breve Bb99, and Propionibacterium freudenreichii ssp. shermanii JS) | NO | 102 |
No refers to no test or no research.
The differences in the gut microbiota composition precede the manifestation of eczema. Numerous studies have extensively investigated how specific species are involved in progressions of eczema (Fig. 1)
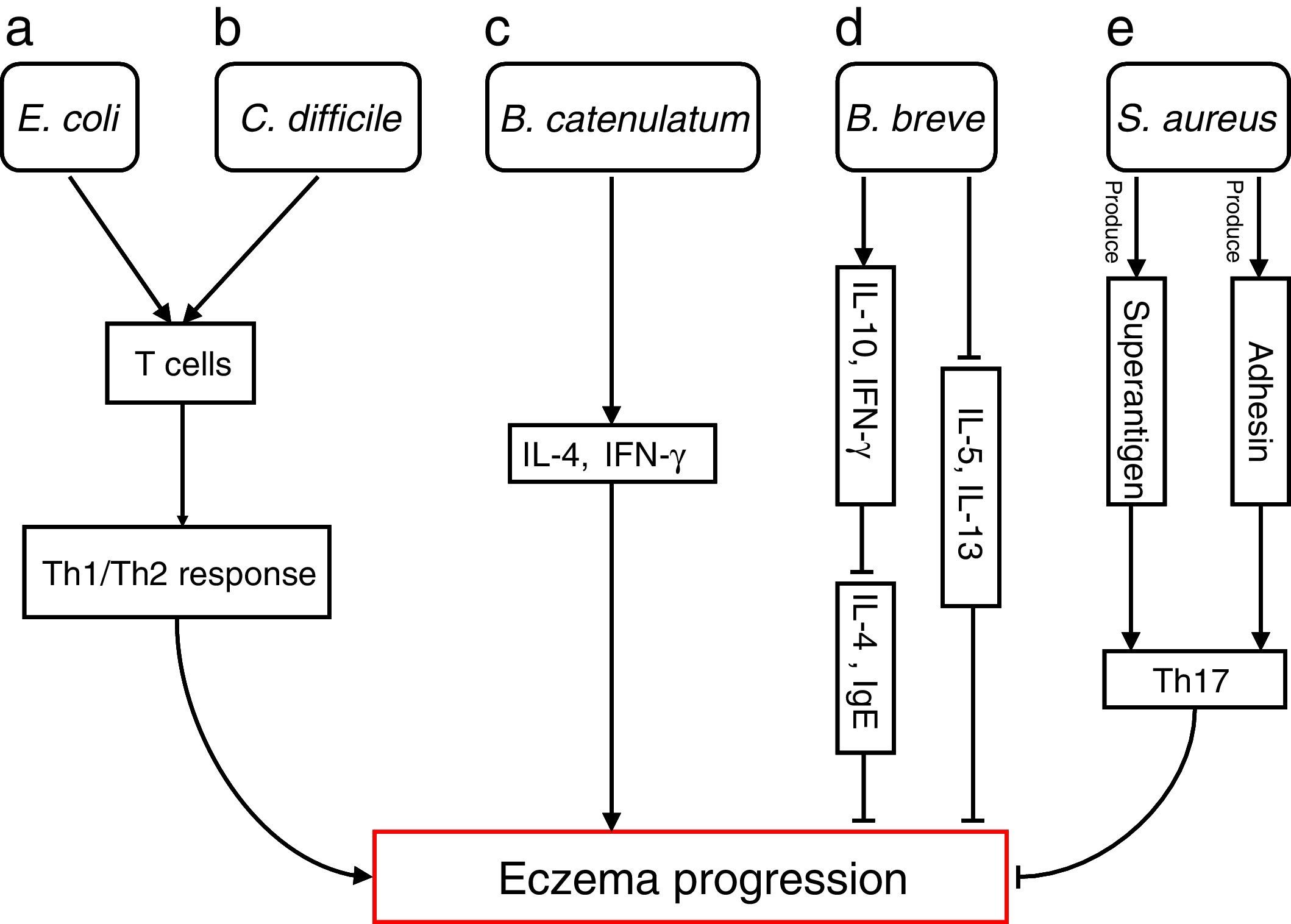
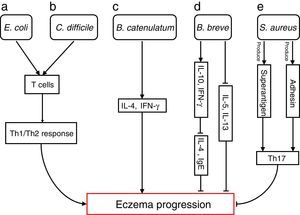
Bacterial species involved in eczema progression. a: E. coli may mediate a Th1/Th2 response by affecting T cells; b: C. difficile may mediate a Th1/Th2 response by affecting T cells; c: B. catenulatum may induce IL-4 and IFN-γ production; d: B. breve may suppress the production of Th2 cytokine (IL-4) and IgE by inducing the secretion of regulatory (IL-10) and Th1 (IFN-γ) cytokines, and reduce allergen-induced Th2 (IL-5and IL-13) responses; e: S. aureus may elicit a Th17 response. Abbreviations: Th1: T helper 1; Th2: T helper 1; IL: Interleukin; IFN-γ: Interferon-γ; IgE: immunoglobulin E; Th17: T helper 17.
Escherichia coli was significantly higher in infants who were going to develop atopic eczema compared with healthy controls.56 Previously, one study reported the number of E. coli to be positively correlated with total serum IgE levels in infants with eczema, indicating that the presence of E. coli is associated with the extent of atopic sensitisation.48E coli (for example, due to LPS) may evoke an inflammatory response in the gut leading to increased allergen uptake and thereby greater atopic sensitisation in infants with atopic eczema.
E. coli57 and Clostridium difficile57,58 were significantly positive associated with a risk of developing eczema. The presence of E. coli and C. difficile could induce a decrease of other beneficial bacteria, which could result in reduced induction of Treg cells by these beneficial bacteria leading to immune dysregulation. In the absence of optimal levels of immune regulation, an individual may develop a Th1 (such as Crohn's disease or autoimmunity) or Th2 (such as atopic diseases) mediated inflammatory disorder. Secondly, E. coli and C. difficile could have a direct effect on the production of cytokines by antigen-presenting cells, thereby affecting the differentiation of T cells.59 Another hypothesis is that E. coli and/or C. difficile increase the intestinal permeability (for instance by the production of toxins). This increased permeability of the intestinal barrier could facilitate the penetration of innocuous antigens and subsequent sensitisation.60 Indeed it has been shown that C. difficile toxins A and B compromise the intestinal cell barrier.61,62 Furthermore, increased intestinal permeability has been described in patients with food allergies, eczema and asthma compared with healthy subjects.63–66
B. catenulatum and B. breve colonisation can influence the development of eczema in infants.67 Namely, B. catenulatum was associated with a higher risk of developing eczema. However, B. breve was associated with a lower risk of eczema. The mechanisms through which these bifidobacteria exert their effects are unknown. The immune effects of exposure to microorganisms are both species and strain dependent, for example the capacity to induce FoxP3 Treg cells68 and stimulate the production of different pro- and anti-inflammatory cytokines.68,69B. catenulatum is reported to be a strong inducer of IL-4 and IFN-γ production.70B. breve induces the production of regulatory and Th1 cytokines. Inoue et al.,71 found B. breve M-16V treatment could suppress the production of Th2 cytokine (IL-4) and IgE by inducing the secretion of regulatory (IL-10) and Th1 (IFN-γ) cytokines in a murine model of allergic inflammation. Similarly, synbiotic (B. breve M-16V and prebiotics) supplement could reduce allergen-induced Th2 (IL-4, IL-5and IL-13) responses72 in asthmatic adults. Taken together, the development of infant eczema can be influenced by modulation of specific bifidobacteria patterns in early life, depending on their immunomodulatory properties.
Staphylococcus aureus can produce a variety of T-cell-activating enterotoxins, called superantigens.73 Gut colonisation by S. aureus strains carrying a certain combination of superantigen and adhesin genes was negatively associated with subsequent development of atopic eczema.73 Such strains may provide stimulation and promote maturation of the infantile immune system. Thus, S. aureus could have a protective effect through the broad immune stimulation afforded by this bacterium. Mucosal colonisation by S. aureus might elicit a Th17 response74 strengthening the skin and mucosal barriers. This may, in turn, reduce the risk of sensitisation and entering into the atopic march. To speculate, exposure of the newborn infant to S. aureus superantigens with limited pathogenic potential, such as SElM, might provide a method to prevent the development of an atopic phenotype.73
Probiotics/prebiotics therapyProbiotics are widely used in medical application to prevent or treat many diseases, such as diarrhoea, obesity, in particular immune disorders like eczema, allergy and eczema. A great number of studies have testified that modulating gut microbiota may be an effective strategy to cure and maintain eczema. The therapeutic effects of probiotics on eczema have also been confirmed in experimental subjects.
Lactobacilli, not only can enhance release of anti-inflammatory factors,75–77 but also appears to protect against the invasion of pathogenic bacteria.78 In a double-blind, placebo-controlled randomised intervention trial, infants with a high risk for eczema were fed cereals with or without Lactobacillus F19.79 The risk of eczema significantly decreased in the probiotic groups compared placebo groups. Meanwhile, the interferon-γ (IFN-γ)/interleukin 4 (IL4) mRNA ratio (the Th1/Th2 ratio) was significantly higher in the probiotic than the placebo group. These results suggested that Lactobacillus F19 may have effect on the T cell-mediated immune response. Another study randomly assigned women to take L. rhamnosus HN001, or placebo daily at gestation week 35 until six months of breastfeeding, and their infants were with high risk for eczema randomly assigned to receive the same treatment from birth to two years.80,81 Infants receiving L. rhamnosus had a significantly reduced risk of eczema. Marlow et al.,82 found that HN001 interacted with Toll-like receptor (TLR) that resulted in a significantly reduced risk of eczema. The main role of TLRs is as pattern recognition receptors, such that TLRs are expressed on T cells and involved in maintaining the balance between Th1 and Th2 immune responses. In addition, Gill et al.83 found mice fed HN001 had significantly higher IFN-γ levels than controls. HN001 may have a protective effect of HN001 against eczema by influencing cytokine production. More recently, children aged 6–18 months with moderate or severe eczema treated with L. fermentum VRI-033PCC,84 showed a significant reduction in eczema scores compared to placebo. Similarly, the mothers received L. reuteri ATCC 55730 daily from gestational week 36 until delivery. Their babies then continued with the same product from birth until 12 months of age and were followed up for another year.85 The L. reuteri group had significantly less IgE-associated eczema. However, skin prick test reactivity was also significantly less common in the treated than in the placebo group. L. reuteri prevents TNF-a-induced IL-8 expression in murine epithelial cells,86 diminishes inflammatory bowel disease in murine models,87 and induces human IL-10 producing regulatory T cells by modulating dendritic cell function in vitro.88 Another mode of action of L. reuteri could be an indirect effect through an influence on the composition of the intestinal microbiota, as L. reuteri strains produce the antimicrobial metabolite reuterin and inhibit pathogenic bacteria, without inhibiting normal bacterial residents of the gastrointestinal tract in vitro.89 Keeping with the observation, infants with atopic eczema were fed an extensively hydrolysed whey formula with or without Lactobacillus GG.49Lactobacillus GG supplement show a significant improvement of atopic eczema after one month's intervention concomitant with a significant reduction in the concentrations of concentration of faecal α1-antitrypsin tumour necrosis factor-α(TNF-α). Another study showed that probiotics L. rhamnosus GG administered pre- and postnatally for six months to children at high risk of atopic disease reduced the risk of developing atopic eczema later in infancy and childhood compared with that in infants receiving placebo.51,90,91 Similarly, L. rhamnosus GG was given prenatally to mothers who had at least one first-degree relative with atopic eczema for four weeks before expected delivery and to their children, postnatally, for three months.92L. rhamnosus GG significantly reduced the risk of developing atopic eczema. Probiotic administration to the pregnant and lactating mother increased the amount of anti-inflammatory cytokine transforming growth factor-β2 (TGF-β2) in the mother's milk, which was suggested to increase its immune-protective potential and to be associated with a reduction in the risk of atopic eczema during the first two years of life. Transforming growth factor β2 (TGF-β2) is considered a key immunoregulatory factor in promoting IgA production and induction of oral tolerance.93,94 Viljanen et al.95 found that in infants with IgE-associated atopic eczema–dermatitis syndrome (AEDS), treatment with LGG induced significantly higher C-reactive protein levels than in the placebo group, concomitantly, IL-6 levels significantly increased after treatment with LGG. Isolauri et al. also reported that oral administration of Lactobacillus GG or Bifidobacterium lactis Bb-1250 in infants manifested atopic eczema significantly reduce the extent, severity and subjective symptoms of atopic eczema, in parallel with a significant reduction in the concentration of soluble CD4 (sCD4) in serum and eosinophilic protein X (EPX) in urine. Reduction of soluble CD4 is a marker of T-cell activation. Soluble CD4 has been found to be elevated in several diseases associated with chronic inflammation,96 while urinary EPX has been shown to reflect the activity of allergic inflammation in childhood asthma.97
The possible influences of probiotic Bifidobacterium bifidum on infants with eczema was evaluated. B. bifidum supplementation significantly reduces the Scoring Atopic Dermatitis (SCORAD) index of infants with eczema as compared with prior to treatment and the controls.98 Further study found that B. bifidum supplementation significantly enhances the levels of B. bifidum in the intestine. B. bifidum colonise in the intestinal tract and resist exogenous pathogens, so as to enhance the immune status of the body. B. bifidum also have a number of other roles, including improving the barrier function of the intestinal immune system, regulating the immune response and reducing the production of inflammatory cytokines and the inflammatory response.99
A mixture of probiotics has also been used for the treatment of eczema. Rautava et al.,100completed a study in which mothers with allergic disease and atopic sensitisation were randomly assigned to receive (1) Lactobacillus rhamnosus LPR and Bifidobacterium longum BL999 (LPR+BL999), (2) L. paracasei ST11 and B. longum BL999 (ST11+BL999), or (3) placebo, beginning two months before delivery and during the first two months of breast-feeding. The infants were followed until the age of 24 months. Probiotic supplementation significantly reduced the risk of developing eczema. Keep with the observation, a mixture of probiotics (Bifidobacterium bifidum, Bifidobacterium lactis, and Lactococcus lactis) was prenatally administered to mothers of high-risk children (i.e. positive family history of allergic disease) and to their offspring for the first 12 months of life.101 Parental-reported eczema during the first three months of life was significantly lower in the intervention group compared with placebo. In addition, the intervention group was significantly more frequently colonised with higher numbers of Lactococcus lactis. Furthermore, at age three months, in vitro production of IL-5 was significantly decreased in the probiotic-group compared with the placebo-group. Similarly, pregnant women carrying high risk children to use the probiotic mixture (Lactobacillus rhamnosus GG (ATCC 53103); L rhamnosus LC705 (DSM 7061); Bifidobacterium breve Bb99 (DSM 13692); and Propionibacterium freudenreichii ssp. shermanii JS (DSM 7076)) or a placebo for two to four weeks before delivery, their infants received the same probiotics plus galacto-oligosaccharides or a placebo for six months.102 Probiotic treatment significantly reduced eczema and atopic eczema. The abundance of Lactobacilli and bifidobacteria was significantly higher in the guts of supplemented infants. Interestingly, in infants with eczema, the same combination of probiotic bacteria as used in this study induced an significant increase in plasma IL-10 levels.95 Primary prevention of eczema by perinatal administration of probiotic bacteria indeed involves modulation of the early colonisation of the intestinal microbiota, which may result in modulating the development and maturation of the infants immune system. Gut microbiota contact directly with extensions of dendritic cells, which orchestrate the mucosal immune homeostasis. Commensal bacteria stimulate the innate immune system and contribute to the generation of regulatory lymphocytes, which, through IL-10 and TGF-β, establish and maintain mucosal immune tolerance.103 Modulation of the immune response via interaction with intestinal dendritic cells with subsequent effects on T-cell differentiation and induction of regulatory T cells has been suggested.88 Furthermore, recognition of commensal bacteria by TLRs on intestinal epithelial cells and cells of the mucosal immune system is essential for intestinal (immune) homeostasis.104,105 Probiotic signalling through TLRs may contribute to maintaining mucosal and intestinal homeostasis and thereby preventing eczema. Above all, probiotics intervention might be a potential effective approach in the treatment of eczema via restoring gut microbiota. Therapies that may most efficiently bring the disease under control are still being sought.
Concluding remarksThe human intestinal hosts possess trillions of microorganisms, also collectively known as the bacterial flora. An increasing number of studies are progressively unravelling the fascinating interaction between hosts and microbiota.106–108 More and more evidence indicates that intestinal microbiota exert a critical function in keeping healthy and the presence of eczema. Its mechanism, however, remains elusive despite promising results from previous studies. The specific strains among commensal gut bacteria may exert either a phylactic or morbific effect into eczema progression. Additionally, previous series completed in both animal and human models demonstrated that an effective strategy of preventing and managing eczema might target on gut microbiota. Probiotics/prebiotic can confer health by modulating the composition of gut microbiota and restoring the physiological bacterial flora. Many studies have provided a compelling rationale for exploring the oral probiotics administered as adjunctive therapies to eczema. That being said, limited data are available in this area amid, and the relevant scientific work is still at the early stage. A lot of studies need to be carried out in the future, as below. Initially, a host provides a large and complex environment for gut bacterial flora. It is as yet unclear whether the alteration of intestinal microbiota contributes to the development of eczema or its presence reflects the primary cause of changes in gut microbiota. There is a need for a profound understanding of the interactive mechanism between intestinal microbiota and host. Of note, a great number of studies need to be completed in diverse populations or mammal models and various types of food. As such, it is singularly critical to offer an entirely novel approach to cure diseases for sound health through monitoring and controlling gut microbiota. Compared with developing novel drugs for inflammation, it might cost less to seek novel approaches, such as monitoring and manipulating human gut microbiota if required using probiotics and/or prebiotics (non-digestible food additives triggering the development and/or activity of bacteria). The opportunity exists to develop updated probiotics in accordance with the interaction between specific microbiota and eczema. In addition, it is plausible to develop probiotics from intestinal microbiota in healthy groups, for instance, faecal transplant as a therapeutic strategy has whetted more appetite. Similarly, it is significant to determine how prebiotics and/or probiotics change the constitution of intestinal microbiota (reconstructing bacterial flora) and how relative it is to eczema. Up to now, only a fraction of human studies have reported the alteration of gut microbiota in pre- and post-probiotics and/or prebiotics treatment among eczema patients. There is also a need for construction of “Pan-microbiome” studies which may play an essential role in a further understanding of eczema aetiopathogenesis. Most importantly, recent findings were validated in studies of the microbiota at diverse stages of eczema (and in differing at-risk populations). Given few relevant researches in the area, more studies should be carried out to elucidate the interaction between specific microbiota and eczema, and a wealth of well-controlled clinical studies on gut microbiota are required to make sure safety in patients. Synergistic efforts in vivo and in vitro are also to be asked for so as to advance our knowledge in the area, which is promising for its existing potential in biomarker reorganisation and novel therapeutic targets. All in all, we have opened up a completely novel access to the understanding and therapy of eczema and more research is urgently needed in this emerging field.
Ethical disclosuresConfidentiality of dataThe authors declare that no patient data appears in this article.
Right to privacy and informed consentThe authors declare that no patient data appears in this article.
Protection of human subjects and animals in researchThe authors declare that no experiments were performed on humans or animals for this investigation.
FundingNone.
Conflict of interestNone declared.
We thank Yue Cai and Wei Pan for critical review and important intellectual contributions to the final version of the manuscript.