Human bocavirus (HBoV) was recently discovered and identified as an important cause of respiratory infection in young children. However, the relationship between HBoV-bronchiolitis and the development of recurrent wheezing has not yet been established.
ObjectiveWe designed this study in order to describe the mid-term outcome, regarding the development of recurrent wheezing and asthma of HBoV-bronchiolitis patients and to compare it with RSV-bronchiolitis infants.
MethodsWe studied 80 children (10 with HBoV and 70 with RSV infection), currently aged ≥4 years and previously hospitalised during the seasons 2004–2009 due to acute bronchiolitis. Epidemiological and clinical data were collected through structured clinical interviews at the follow-up visit. Spirometry and skin prick tests to common food and inhaled allergens were performed.
ResultsAll HBoV-patients developed recurrent wheezing and half of them had asthma at age 5–7 years. Almost 30% required hospital admission for recurrent wheezing. Asthma (odds ratio (OR)=1.28) and current asthma (OR=2.18) were significantly more frequent in children with HBoV-bronchiolitis than in RSV-bronchiolitis. FEV1 values were 99.2±4.8 in HBoV-group vs. 103±11 in RSV-group, p: 0.09. No differences were found with respect to allergic rhinitis, atopic dermatitis, food allergy, proportion of positive prick tests, and family history of atopy or asthma.
ConclusionsSevere HBoV-bronchiolitis in infancy was strongly associated with asthma at 5–7 years.
Bronchiolitis is one of the leading causes of hospital admission for infants.1 Long-term studies have demonstrated that infants hospitalised with bronchiolitis have a 2- to 3-fold increase in the risk of asthma. To date, most research has focused on respiratory syncytial virus (RSV) and in the last years, in rhinovirus infections.2
Human bocavirus (HBoV) was discovered in 2005 in nasopharyngeal specimens from children with respiratory tract infection,3 and identified as an important causative agent of respiratory infections in young children.4 However, the relationship between HBoV-bronchiolitis and the development of subsequent recurrent wheezing and asthma has not been investigated yet.
We designed this study in order to describe the mid-term outcome, regarding the development of recurrent wheezing and asthma, of HBoV-bronchiolitis patients and to compare it with RSV-bronchiolitis infants.
Study designThis is a sub study of an ongoing prospective investigation on respiratory tract infections in children, funded by FIS (Fondo de Investigaciones Sanitarias – Spanish Health Research Fund) grants n°: PS09/00246 and PI12/0129 and approved by The Medical Ethics Committee of Severo Ochoa Hospital.
The study population consisted of children of at least 4 years of age with a previous history of hospital admission at age 0–24 months due to HBoV or RSV bronchiolitis. To avoid the confounding role of coinfections, patients with dual or multiple infections were excluded. Nasopharyngeal aspirates were obtained and sent to the Influenza and Respiratory Viruses Laboratory at the National Microbiology Center (ISCIII, Madrid). Simple and multiplex reverse transcription-nested PCR assays previously described5–8 were used to assess the viral aetiology of bronchiolitis, including 16 different respiratory viruses or group of viruses.
A total of 738 children less than 2 years were admitted at the secondary public hospital Severo Ochoa (Leganés, Madrid, Spain), between September 2004 and August 2009 due to bronchiolitis. Fifty-four parents refused NPA collection but accepted their children to be included in the clinical study of the 684 analysed samples, 588 (86%) were positive for at least one virus, 421 (71.6%) of which were single infections: 274 RSV, 62 rhinovirus, 31 HBoV, 18 hMPV, 14 parainfluenza, 13 adenovirus and 9 influenza. At the time of the study, 18 HBoV and 225 RSV children were older than 4 years of age. A random sample of 80 RSV-hospitalised bronchiolitis was selected using Excel data analysis function.
Parents of children with bronchiolitis due to single HBoV and single RSV infection were contacted by telephone from June to October 2012 and invited to a follow-up visit. Ten HBoV and 70 RSV patients could be contacted and were finally recruited in the study. Informed consent was obtained from parents or legal guardians.
A clinical interview based on a structured questionnaire was performed, to obtain information regarding wheezing episodes, hospital admissions, use of bronchodilators and maintenance medication for asthma. In addition, information about the presence of atopic dermatitis, allergic rhinitis and food allergy was also collected, as were demographic factors, environmental exposures and family history of respiratory and atopic disease. The researchers were not blinded to the status of the child when the interviews were performed.
Primary care paediatricians were also contacted by phone and asked to review the patients’ electronic records in order to confirm the presence of wheezing episodes, their number and the use of bronchodilator and maintenance medication prescription for asthma. Only the information confirmed by the paediatrician in charge of the patient was taken into account.
Spirometry was performed according to established guidelines9 using a Jaeger MasterScope-PC spirometer (VIASYS HealthCare GmbH, Hochberg, Germany). Forced expiratory volume in first second (FEV1), forced vital capacity (FVC), FEV1/FVC ratio and forced expiratory flow at 50% of FVC (FEF50) were recorded. Measurements were compared to values predicted by standard reference equations10 and expressed as percentages of predicted values. FEV1 and FVC ≥80% of predicted values were considered as normal.
For the estimation of allergic sensitisation, skin prick tests for common inhaled allergens (Dermatophagoides pteronyssimus, D. farinae, grass pollen mix, Olea europaea, Platanus hispanica, cat, dog, Blatella germanica and Alternaria tenuis) were performed using standardised extracts (ALK-Abelló SA, Madrid, Spain). Histamine (10mg/ml) was used as a positive control and 0.9% saline solution as a negative control. Children were advised not to take antihistaminic medication for 1 week before the test. Commercially available lancets were used to prick the epidermis with the allergen extract drops. The tests were read at 15min, and mean wheal diameters were calculated (sum of the longest diameter and its perpendicular one divided by two). A mean wheal diameter of at least 3mm greater than the negative control was taken as positive.
Bronchiolitis was defined as the first episode of expiratory wheezing of acute onset with previous signs of viral respiratory infection in children younger than 2 years. Asthma was defined as at least three episodes of bronchial obstruction confirmed by a paediatrician. Current asthma was defined as recurrent wheezing with at least one episode occurring in the year prior to the follow-up visit.
Statistical analysisValues were expressed as percentages for discrete variables, or as mean and standard deviation and median and interquartile range for continuous variables. Clinical characteristics and laboratory variables were compared using the Student t test, the Mann–Whitney U test, the χ2 test, and Fisher's exact test, where appropriate. A two-sided value of P=0.05 was considered to be statistically significant. All analyses were performed with the Statistical Package for the Social Sciences (SPSS), Version 20.
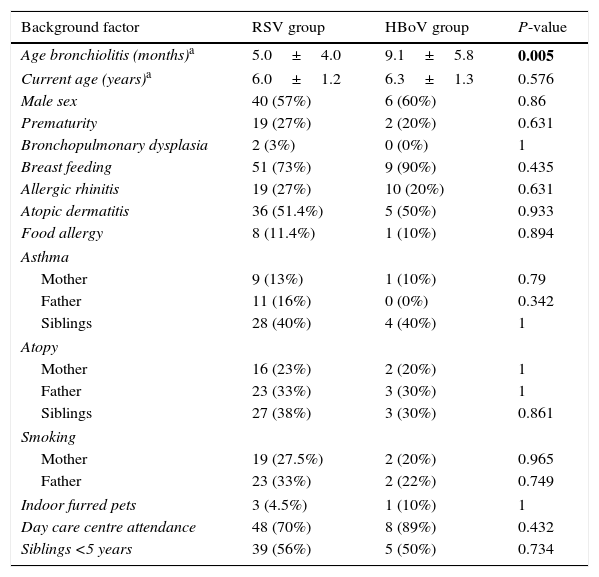
ResultsAll children included were older than 4 years, with a mean age at the time of the study of 6.3±1.3 years for HBoV vs. 6.0±1.2 years for the RSV group (p: 0.576). The basic characteristics of the 80 included children are presented in Table 1. HBoV and RSV groups were similar for 19 out of 20 background factors: sex; prematurity; bronchopulmonary dysplasia; breast feeding; allergic rhinitis; atopic dermatitis; food allergy; family history of asthma and atopy; exposure to passive tobacco smoke; indoor furred pets; day care centre attendance; and siblings <5 years. However, HBoV-patients were significantly older at admission than RSV infants.
Evaluation of background factors in children with RSV and HBoV bronchiolitis.
| Background factor | RSV group | HBoV group | P-value |
|---|---|---|---|
| Age bronchiolitis (months)a | 5.0±4.0 | 9.1±5.8 | 0.005 |
| Current age (years)a | 6.0±1.2 | 6.3±1.3 | 0.576 |
| Male sex | 40 (57%) | 6 (60%) | 0.86 |
| Prematurity | 19 (27%) | 2 (20%) | 0.631 |
| Bronchopulmonary dysplasia | 2 (3%) | 0 (0%) | 1 |
| Breast feeding | 51 (73%) | 9 (90%) | 0.435 |
| Allergic rhinitis | 19 (27%) | 10 (20%) | 0.631 |
| Atopic dermatitis | 36 (51.4%) | 5 (50%) | 0.933 |
| Food allergy | 8 (11.4%) | 1 (10%) | 0.894 |
| Asthma | |||
| Mother | 9 (13%) | 1 (10%) | 0.79 |
| Father | 11 (16%) | 0 (0%) | 0.342 |
| Siblings | 28 (40%) | 4 (40%) | 1 |
| Atopy | |||
| Mother | 16 (23%) | 2 (20%) | 1 |
| Father | 23 (33%) | 3 (30%) | 1 |
| Siblings | 27 (38%) | 3 (30%) | 0.861 |
| Smoking | |||
| Mother | 19 (27.5%) | 2 (20%) | 0.965 |
| Father | 23 (33%) | 2 (22%) | 0.749 |
| Indoor furred pets | 3 (4.5%) | 1 (10%) | 1 |
| Day care centre attendance | 48 (70%) | 8 (89%) | 0.432 |
| Siblings <5 years | 39 (56%) | 5 (50%) | 0.734 |
Significant values are in bold.
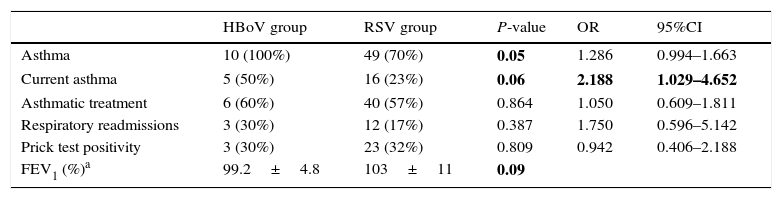
Respiratory complaints developed after bronchiolitis admissions are shown in Table 2. Asthma was diagnosed more frequently in children with history of HBoV bronchiolitis than RSV bronchiolitis. Current asthma was also more prevalent in the HBoV than in RSV group. The rate of respiratory readmission due to recurrent wheezing was double in HBoV children although the difference did not reach statistical significance. On the other hand, although HBoV-infants were older than RSV at admission, the age of diagnosis of bronchiolitis was not associated with later asthma.
Respiratory evolution after HBoV and RSV bronchiolitis.
| HBoV group | RSV group | P-value | OR | 95%CI | |
|---|---|---|---|---|---|
| Asthma | 10 (100%) | 49 (70%) | 0.05 | 1.286 | 0.994–1.663 |
| Current asthma | 5 (50%) | 16 (23%) | 0.06 | 2.188 | 1.029–4.652 |
| Asthmatic treatment | 6 (60%) | 40 (57%) | 0.864 | 1.050 | 0.609–1.811 |
| Respiratory readmissions | 3 (30%) | 12 (17%) | 0.387 | 1.750 | 0.596–5.142 |
| Prick test positivity | 3 (30%) | 23 (32%) | 0.809 | 0.942 | 0.406–2.188 |
| FEV1 (%)a | 99.2±4.8 | 103±11 | 0.09 |
Significant values are in bold.
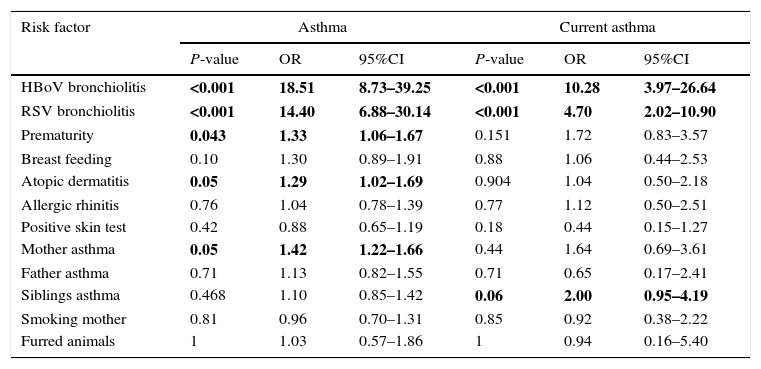
Several possible hereditary and environmental factors for the development of asthma and current asthma bronchial obstructive symptoms were evaluated (Table 3). The risk of asthma was greatly increased for children with HBoV bronchiolitis, RSV bronchiolitis, prematurity, atopic dermatitis and mother with asthma. Similarly HBoV and RSV bronchiolitis and siblings with asthma were significantly associated to current asthma.
Univariate test of various possible risk factors for asthma and current asthma.
| Risk factor | Asthma | Current asthma | ||||
|---|---|---|---|---|---|---|
| P-value | OR | 95%CI | P-value | OR | 95%CI | |
| HBoV bronchiolitis | <0.001 | 18.51 | 8.73–39.25 | <0.001 | 10.28 | 3.97–26.64 |
| RSV bronchiolitis | <0.001 | 14.40 | 6.88–30.14 | <0.001 | 4.70 | 2.02–10.90 |
| Prematurity | 0.043 | 1.33 | 1.06–1.67 | 0.151 | 1.72 | 0.83–3.57 |
| Breast feeding | 0.10 | 1.30 | 0.89–1.91 | 0.88 | 1.06 | 0.44–2.53 |
| Atopic dermatitis | 0.05 | 1.29 | 1.02–1.69 | 0.904 | 1.04 | 0.50–2.18 |
| Allergic rhinitis | 0.76 | 1.04 | 0.78–1.39 | 0.77 | 1.12 | 0.50–2.51 |
| Positive skin test | 0.42 | 0.88 | 0.65–1.19 | 0.18 | 0.44 | 0.15–1.27 |
| Mother asthma | 0.05 | 1.42 | 1.22–1.66 | 0.44 | 1.64 | 0.69–3.61 |
| Father asthma | 0.71 | 1.13 | 0.82–1.55 | 0.71 | 0.65 | 0.17–2.41 |
| Siblings asthma | 0.468 | 1.10 | 0.85–1.42 | 0.06 | 2.00 | 0.95–4.19 |
| Smoking mother | 0.81 | 0.96 | 0.70–1.31 | 0.85 | 0.92 | 0.38–2.22 |
| Furred animals | 1 | 1.03 | 0.57–1.86 | 1 | 0.94 | 0.16–5.40 |
Significant values are in bold.
Valid spirometries were obtained in seven HBoV and 40 RSV children, with normal values in all of them. However, significant lower values of FEV1 were observed in HBoV children and in asthmatic patients.
Twenty-one patients (30%) in the RSV group and 4 (40%) in the HBoV group refused prick test performance (p: 0.523). More asthmatic patients, 44 (75%), accepted prick test than non-asthmatic ones, 11 (52.4%), p: 0.06. However, when the tests were done, a similar proportion of positive results were found in HBoV and RSV group (p: 0.887) and in asthmatic vs. non-asthmatic patients (p: 0.418).
DiscussionOur results suggest that severe HBoV bronchiolitis is followed by recurrent wheezing and asthma in a significant proportion of cases. All HBoV patients included in this study developed recurrent wheezing, and half of them had asthma. Furthermore, almost one out of three children required rehospitalisation due to bronchial obstruction.
RSV is the major pathogen responsible for bronchiolitis, whereas HBoV accounts for approximately 10% of cases.11 The relationship between severe RSV-bronchiolitis and recurrent wheezing has been widely investigated. It is estimated that almost 22% of infants who are hospitalised due to RSV infections will develop asthma in the first 5 years of life.12 Most studies that have compared the mid-term outcome of severe RSV and non-RSV bronchiolitis have shown a higher risk of asthma in children with non-RSV infections.13 Among these non-RSV cases, we find not only rhinovirus, but also other viruses such as human metapneumovirus and HBoV. However, no previous study has specifically addressed the outcome of severe HBoV-bronchiolitis. There is only one recently published study that suggests that more than one quarter of children admitted to hospital due to HBoV infections develop recurrent wheezing. Nevertheless, as the study included children hospitalised up to the age of 5 years, many of them did not have bronchiolitis. Besides, coinfections were not excluded and patients were followed up for only 1 year.14 On the other hand, we have only included infants with bronchiolitis due to single HBoV infection and evaluated them at least 4 years after hospital admission. Half of the children included in our study developed asthma, whereas asthma prevalence in children aged 6–7 years in our country is 9.5%, according to data from the International Study of Asthma and Allergies in Childhood (ISAAC) Phase Three.15 We did not find any lung function abnormalities in former HBoV-bronchiolitis patients.
The main limitation of this study is its small sample size, which is due to several circumstances: the recent discovery of the virus, the relatively few cases of HBoV-bronchiolitis, its tendency to infect older children and the high coinfection rate. HBoV affects mainly children aged 6–24 months, who are at lower risk for hospital admission than infants less than 6 months of age. Coinfection has been documented in up to 83% of respiratory samples.16 We excluded coinfections in our study, which accounted for 67% of HBoV infections, to better analyse the outcome of HBoV-bronchiolitis. On the other hand, to correctly diagnose an HBoV infection, it would be necessary to screen clinical samples from the respiratory tract and a corresponding serum sample. However blood samples from young infants are not easily available in clinical practice. Nevertheless, our patients were infected only by HBoV and presented simultaneously respiratory symptoms.
Our study is the first to suggest that severe HBoV-bronchiolitis in infancy is strongly associated with recurrent wheezing. Until these data are confirmed in larger studies, we recommend a close follow up of children with severe non-RSV bronchiolitis.
Ethical approvalMedical Ethics Committee, Hospital Severo Ochoa.
Ethical disclosuresPatients’ data protectionThe authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors declare that no patient data appears in this article.
Protection of human subjects and animals in researchThe authors declare that no experiments were performed on humans or animals for this investigation.
Conflict of interestNone declared.
This study has been partially supported by FIS (Fondo de Investigaciones Sanitarias – Spanish Health Research Fund) Grants N°: PI09/00246 and PI12/012.