Low levels of serum CC16 were reported in asthmatic adults, but the studies on children were scarce and conflicting. The aim of this study was to compare serum CC16 levels in pre-school children with recurrent wheezing assessed using an asthma predictive index (API).
MethodsWe performed a case-control study based on API, with all enrolled pre-school children who had recurrent wheezing episodes (>3 episodes/last year confirmed by a physician) and had presented at one paediatric clinic in Santiago, Chile. The population was divided according to stringent API criteria into positive or negative.
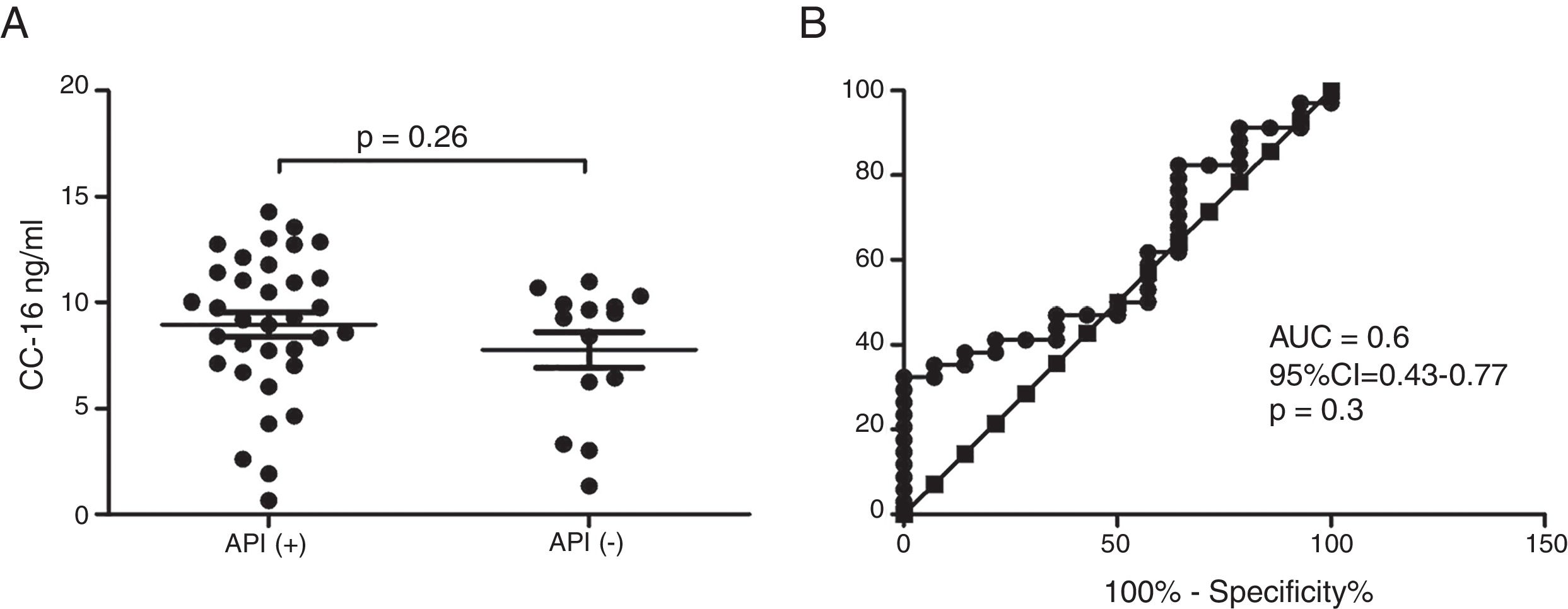
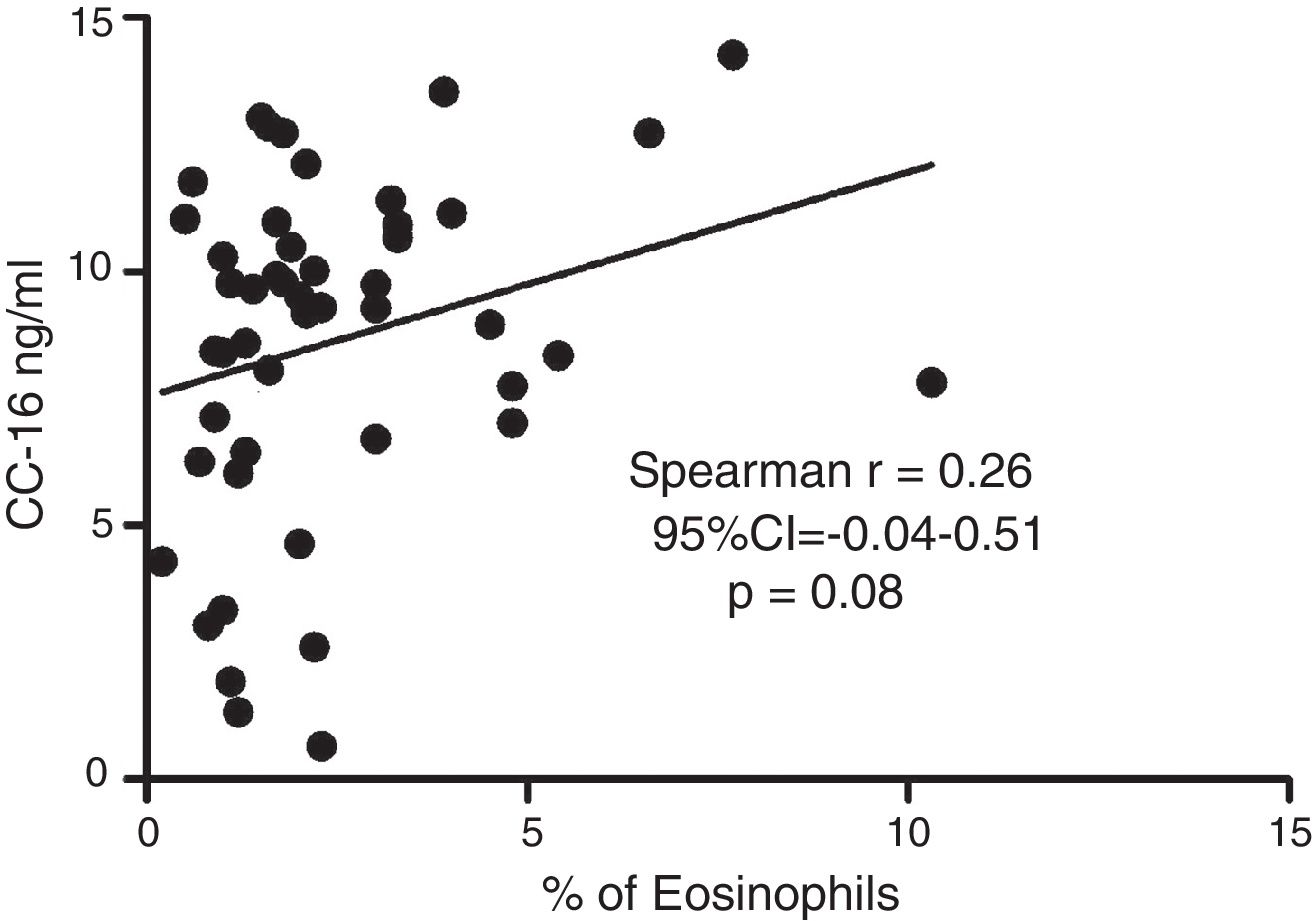
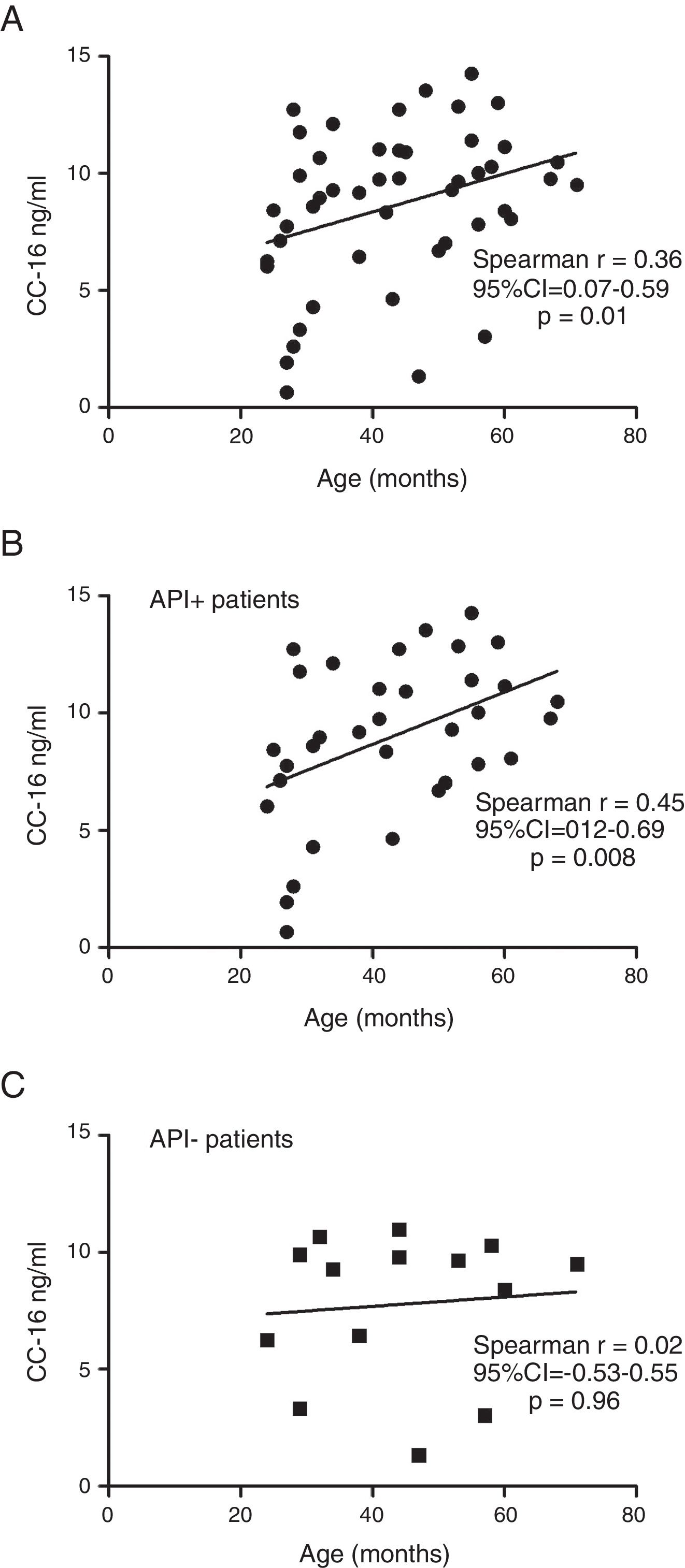
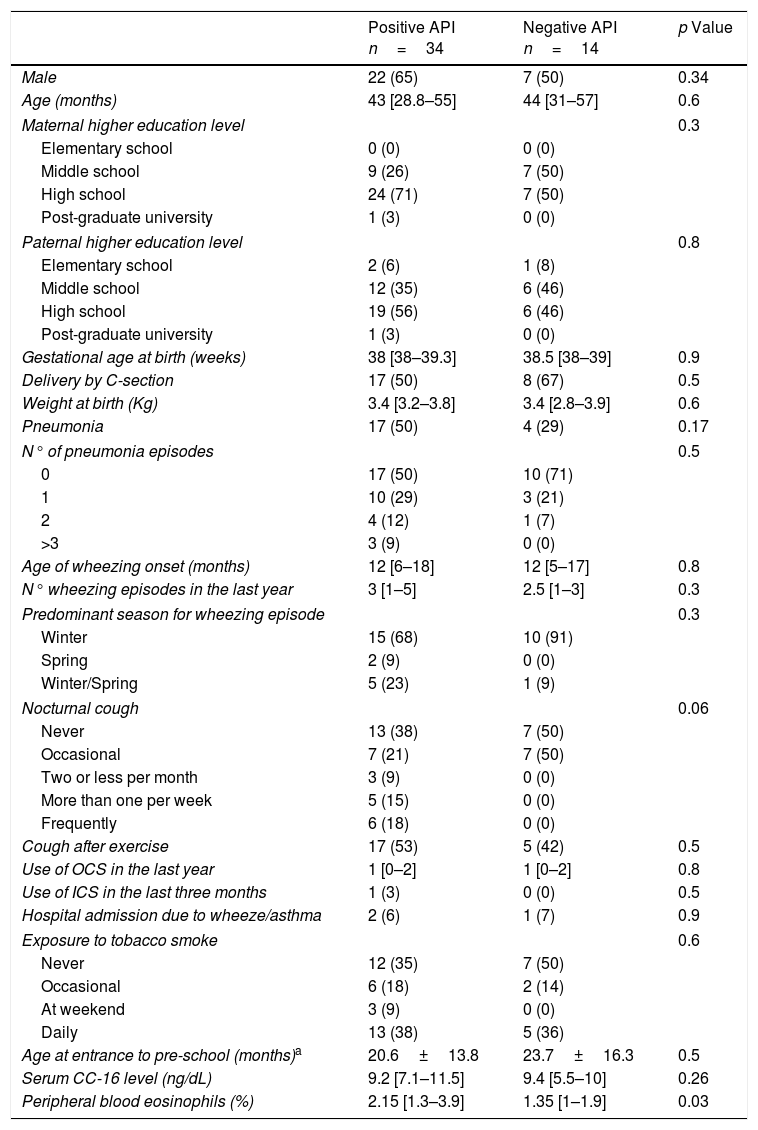
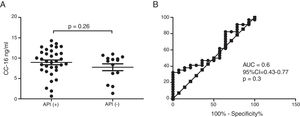
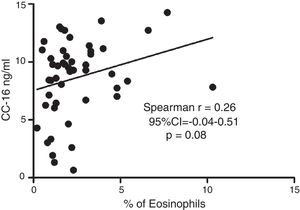
ResultsIn a one-year period, 60 pre-schoolers were enrolled. After excluding 12, 48 pre-schoolers remained (27 males, age range from 24 to 71 months) and completed the study; 34 were API positive and 14 were API negative. There were no significant differences in demographics between groups. The level of serum CC16 levels for pre-schoolers with a positive API and negative API were (median 9.2 [7.1–11.5] and 9.4 [5.5–10], p=0.26, respectively). The area under the curve for the serum CC16 levels to predict a positive API was 0.6, 95% CI [0.43–0.77], p=0.3. A correlation between serum CC16 levels and age was found (r=0.36 [0.07–0.59], p=0.01], but not between serum CC16 levels and peripheral eosinophils blood.
ConclusionThere was no evidence that serum CC16 levels played a role in recurrent wheezing and a positive API in pre-school children. More studies are needed to confirm this finding.
Recurrent wheezing in pre-school age is frequently the first sign of asthma that susceptible children exhibit on presentation. However, many children who wheeze during early life do not go on to develop childhood asthma.1 Based on the epidemiological data on the natural history and temporal patterns of wheezing, several childhood wheezing phenotypes have been described. However, the use of these “epidemiological” phenotypes of wheezing is limited, since they can only be identified retrospectively.2 In order to address that problem, several asthma predictive methods have emerged.3 The Asthma Predictive Index (API)4 is the most widely used and the only one that fulfils all the steps for clinical prediction indexes5 e.g. development,4 validation/updating,6–8 impact9–12 and implementation.13–16 The API, which relates to atopic asthma inception, is simple and cheap, and its major strength is its good positive likelihood ratio ∼7.4 and high specificity (∼97%).17
Clara cell secretory protein (CC16) is a 16kDa protein secreted by the non-ciliated, non-mucous secretory cells of the tracheobronchial epithelium, prostate, endometrium, and urogenital tract.18–22 CC16 appears to function as an anti-inflammatory agent in both the respiratory and urogenital tracts.18 CC16 inhibit both neutrophil and monocyte chemotaxis, thus preventing infiltration of inflammatory cells.23 Moreover, CC16 is a potent inhibitor of phospholipase A2 activity, which limits the metabolism of arachidonic acid and the synthesis of prostaglandin and leukotriene mediators.24 CC16 is thought to be a major component of the extracellular lining fluid of airways.25 In an experimental study in mice, CC16 airway epithelial cells substantially contribute to the infiltration of eotaxin-responsive CCR3+ immune cells and augment the allergic immune response in the lung.26
It was reported that the levels of CC16 protein are reduced in the bronchoalveolar lavage fluid from asthmatic adults.27 In addition, the CC16 protein expression is downregulated in the small airways and reduced in the serum in asthmatic adults.28–30 However, the literature on CC16 in asthmatic children is scarce.31–32 Indeed, no study had been exclusively performed on pre-schoolers as yet.
The objective of this study was to assess if pre-schoolers with recurrent wheezing episodes and positive API differ in serum CC16 levels than those with negative API, and if peripheral eosinophils correlate with serum CC16 levels. Our hypothesis was that pre-schoolers with positive API would have different serum CC16 levels than those with negative API.
MethodsIn this case–control study, a convenience sample of sixty-two pre-schoolers (2–5 years of age) with recurrent wheezing episodes (more than 3 episodes in the last year) who attended the outpatient paediatric clinic at Pontificia Universidad Catolica de Chile were recruited from March 2015 to March 2016. The study was previously approved by the Ethical Committee at the Pontificia Universidad Catolica de Chile (# 14-048). Informed consent was obtained from the parents or legal guardians before patient inclusion.
The inclusion criteria were: recurrent wheezing (≥3 episodes/last year with diagnosis by a paediatrician) without inhaled corticosteroids (ICS) treatment in the last month and without leukotriene receptor antagonist (LTRA) in the last two weeks. Exclusion criteria were patients with other chronic respiratory illness e.g. cystic fibrosis, bronchopulmonary dysplasia, post-infection bronchiolitis obliterans, cardiac or pulmonary malformations, and acute respiratory infection in the last three weeks.
A detailed questionnaire was completed at enrolment. It included demographic characteristics, parental history of asthma and allergic diseases, rhinitis, atopic dermatitis, parental higher educational degree, history of wheezing episodes, onset and their severity, cough at night and after exercise, exposure to tobacco smoke, and day-care attendance. The same day, a peripheral blood sample was obtained to assess eosinophil counts and serum CC16 levels. Serum was stored at −20°C before measurement of CC16 levels. CC16 was determined by commercially available ELISA kit (BioVendor, Modrice, Czech Republic) and expressed as ng/mL.
Using the data from the questionnaire and peripheral blood eosinophil count, patients were divided into two groups: positive API and negative API. We used the stringent API definition, e.g. positive API was considered if they had one major (history of a physician diagnosis of asthma or physician diagnosis of atopic dermatitis) or two minor criteria (physician diagnosis of allergic rhinitis, wheezing apart from colds or peripheral blood eosinophilia >4%).4
Statistical analysisWe compared pre-schoolers with positive API vs. negative API. Continuous variables with normal distribution were described as a mean±standard deviation (SD), and compared using T-tests. Continuous variables without normal distribution were described as a median [25–75 percentile], and compared with non-parametric tests (Mann–Whitney). Categorical variables were described in contingency tables and compared using chi2 with Fisher's exact test. A bivariate risk analysis was performed by calculating odds ratio (OR) with 95% confidence interval (CI). Spearman's rho correlation between the serum CC16 levels and peripheral blood eosinophils was performed. The receiver operational curve (ROC) analyses were performed to investigate the capacity of serum CC16 levels to predict a positive API. The overall accuracy of the test was measured as the area under the ROC curve. Statically significant differences were considered for a p value <0.05. All statistical analyses were performed using GraphPad Prism V.5.
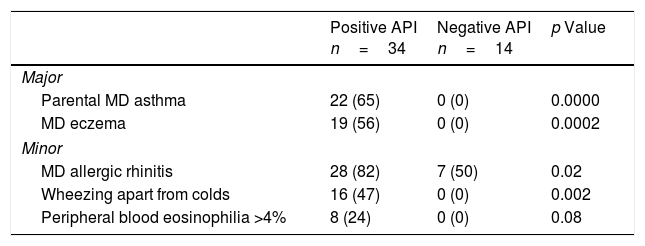
ResultsSixty pre-schoolers were initially enrolled, 12 declined to participate (mainly because they did not fulfil inclusion criteria or refused the blood sample extraction). Of the remaining 48 pre-schoolers, 27 (56%) were males, the overall age range was 24–71 months, 34 had a positive API and 14 had a negative API. The distribution of major and minor API criteria between children with positive and negative API are shown in Table 1.
Presence of major and minor criteria of asthma predictive index between groups.
| Positive API n=34 | Negative API n=14 | p Value | |
|---|---|---|---|
| Major | |||
| Parental MD asthma | 22 (65) | 0 (0) | 0.0000 |
| MD eczema | 19 (56) | 0 (0) | 0.0002 |
| Minor | |||
| MD allergic rhinitis | 28 (82) | 7 (50) | 0.02 |
| Wheezing apart from colds | 16 (47) | 0 (0) | 0.002 |
| Peripheral blood eosinophilia >4% | 8 (24) | 0 (0) | 0.08 |
API, asthma predictive index; MD, medical diagnosis.
There were no significant differences in terms of demographic and perinatal characteristics, maternal education, day-care entrance, and tobacco exposure between those patients with positive and negative API (Table 2). There was some evidence that pneumonia was more prevalent in the positive than the negative API group but this did not reach statistical significance (50% vs. 29%, p=0.17). Also, no significant difference was observed in terms of the characteristics of wheezing episodes (age of onset, seasonality, number of oral steroids bursts, and hospitalisations). Pre-schoolers with a positive API had some evidence of more cough at night than negative API patients (62 vs. 59%, p=0.06, respectively). Only 3% of pre-schoolers with positive API and none in the negative API group received ICS in the past as part of their management (Table 2).
Demographic and clinical characteristic of pre-schoolers with positive and negative asthma predictive index.
| Positive API n=34 | Negative API n=14 | p Value | |
|---|---|---|---|
| Male | 22 (65) | 7 (50) | 0.34 |
| Age (months) | 43 [28.8–55] | 44 [31–57] | 0.6 |
| Maternal higher education level | 0.3 | ||
| Elementary school | 0 (0) | 0 (0) | |
| Middle school | 9 (26) | 7 (50) | |
| High school | 24 (71) | 7 (50) | |
| Post-graduate university | 1 (3) | 0 (0) | |
| Paternal higher education level | 0.8 | ||
| Elementary school | 2 (6) | 1 (8) | |
| Middle school | 12 (35) | 6 (46) | |
| High school | 19 (56) | 6 (46) | |
| Post-graduate university | 1 (3) | 0 (0) | |
| Gestational age at birth (weeks) | 38 [38–39.3] | 38.5 [38–39] | 0.9 |
| Delivery by C-section | 17 (50) | 8 (67) | 0.5 |
| Weight at birth (Kg) | 3.4 [3.2–3.8] | 3.4 [2.8–3.9] | 0.6 |
| Pneumonia | 17 (50) | 4 (29) | 0.17 |
| N° of pneumonia episodes | 0.5 | ||
| 0 | 17 (50) | 10 (71) | |
| 1 | 10 (29) | 3 (21) | |
| 2 | 4 (12) | 1 (7) | |
| >3 | 3 (9) | 0 (0) | |
| Age of wheezing onset (months) | 12 [6–18] | 12 [5–17] | 0.8 |
| N° wheezing episodes in the last year | 3 [1–5] | 2.5 [1–3] | 0.3 |
| Predominant season for wheezing episode | 0.3 | ||
| Winter | 15 (68) | 10 (91) | |
| Spring | 2 (9) | 0 (0) | |
| Winter/Spring | 5 (23) | 1 (9) | |
| Nocturnal cough | 0.06 | ||
| Never | 13 (38) | 7 (50) | |
| Occasional | 7 (21) | 7 (50) | |
| Two or less per month | 3 (9) | 0 (0) | |
| More than one per week | 5 (15) | 0 (0) | |
| Frequently | 6 (18) | 0 (0) | |
| Cough after exercise | 17 (53) | 5 (42) | 0.5 |
| Use of OCS in the last year | 1 [0–2] | 1 [0–2] | 0.8 |
| Use of ICS in the last three months | 1 (3) | 0 (0) | 0.5 |
| Hospital admission due to wheeze/asthma | 2 (6) | 1 (7) | 0.9 |
| Exposure to tobacco smoke | 0.6 | ||
| Never | 12 (35) | 7 (50) | |
| Occasional | 6 (18) | 2 (14) | |
| At weekend | 3 (9) | 0 (0) | |
| Daily | 13 (38) | 5 (36) | |
| Age at entrance to pre-school (months)a | 20.6±13.8 | 23.7±16.3 | 0.5 |
| Serum CC-16 level (ng/dL) | 9.2 [7.1–11.5] | 9.4 [5.5–10] | 0.26 |
| Peripheral blood eosinophils (%) | 2.15 [1.3–3.9] | 1.35 [1–1.9] | 0.03 |
Numbers were expressed as (%), mean±SD, or median [25–75 percentile] when corresponding.
Serum CC16 levels were not significantly different between pre-schoolers with positive and negative API (9.2 [7.1–11.5] ng/dL vs. 9.4 [5.5–10] ng/dL, p=0.26, respectively), (Table 2 and Fig. 1A). The AUC for the serum CC16 levels for predicting positive API was 0.6, 95% CI [0.43–0.77], p=0.3 (Fig. 1B). No significant correlation was identified between serum CC16 levels, and peripheral eosinophils blood was found (Fig. 2). A significantly positive correlation between age and CC16 was found (r=0.36, [0.07–0.59], p=0.01), but it was primarily due to pre-schoolers with a positive API, (Fig. 3A–C). No differences in CC16 levels compared with different levels of severity of wheezing episodes nor the diagnosis of pneumonia were found (data not shown). Upon excluding those patients with corticosteroid use in the past, no changes in CC16 levels were observed between groups (data not shown).
Serum CC16 levels in pre-schoolers between pre-schoolers with differential API score. (A) Serum CC16 levels (ng/mL) in pre-schoolers with positive and negative API. Data are presented as individual patient's points plus median and 25–75 percentile. Analysis was performed using Mann–Whitney test. (B) Receiver operating curve (ROC) analysis for serum CC16 levels and API Score. AUC is shown.
This study on 48 recurrent wheezing Chilean pre-schoolers showed, contrary to our hypothesis, no significant difference in serum CC16 levels between subjects with a positive or negative API. We found increased serum CC16 levels with age. To our knowledge, this is the first study that compares CC16 serum levels exclusively in a pre-school population.
Only a few studies on asthmatic schoolchildren have been performed. Gioldassi et al.31 compared serum CC16 levels among 24 Greek asthmatic children (0–14 years old) with 27 healthy children. They found significantly lower values in the asthma group compared with healthy children (13.2±8.4ng/ml vs. 27.5±17.6ng/ml). However, no comparisons were made in the pre-school subgroup. Ma et al.32 on 147 Chinese asthmatic children (9–15 years old) showed lower levels of urine CC16 and FVC (but not FEV1) in asthmatic children compared to controls, after adjustment for sex, age, BMI, parental education and smoking status; suggesting that CC16 may be a useful tool or biomarker for investigating lung epithelium integrity among children with asthma or lung injury. A study in healthy Swedish children reported significantly different levels of CC16 in blood according with age (e.g. median levels of 15ng/ml at birth, 96ng/ml at 4 months, 20ng/ml at 18 months, and 7ng/ml at age of 3 years).33 Four-month-old children with a parental history of allergy had similar levels of CC16 as the children with no parental history of allergy (94ng/ml vs. 100ng/ml).33 We found a significant positive correlation between serum CC16 levels with age, particularly among those with a positive API; but no difference on serum CC16 levels between positive (∼type 2 or allergic inflammation) vs. negative API.
Studies on asthmatic adults are conflicting and also scarce. Shijubo et al.28 reported no differences in serum CC16 between atopic and non-atopic asthmatic adults. In addition, asthmatics with a long duration of the illness (>10 years) had lower levels of serum CC16 than those with a less than 10-year history of asthma.29 Ye et al.34 found a correlation between lung function (FEV1/FVC) and serum CC16 levels in asthmatic adults. However, de Burbure et al.35 found no difference in sputum CC16 levels in adults with atopic asthma, atopic rhinitis and non-smoking non-atopic controls.
Recently, Guerra et al.36 assessed longitudinal data on CC16 concentration in serum and decline in FEV1 and incidence of airflow limitation for adults who were free from COPD at baseline in three cohorts. After adjustment for sex, age, height, smoking status and intensity, asthma, and FEV1 baseline, they found an inverse association between serum CC16 concentration and decline in FEV1 in adults. Also, the authors explored in three birth cohorts whether low CC16 concentrations in childhood were predictive for subsequent lung function. They reported that the lowest tertile of CC16 concentration was associated with a subsequent FEV1 deficit up to age 16 years, which was confirmed in children who had never smoked by 16 years.
The CC16 gene is located on chromosome 11q 12–13, a region that has been associated with asthma and atopy in several genome-wide linkage studies.37 Some studies explore the relevance on polymorphism of the CC16 gene. Laing et al.38 in a study performed in severe Australian asthmatic schoolchildren showed that a polymorphism of the CC16 gene homozygous 38AA had a 6.9-fold increased risk of developing asthma and heterozygotes 38AG a 4.2-fold increased risk, independently of age, gender, and tobacco smoke exposure. In contrast, Sengler et al.39 found no association on the CC16*38A allele with asthma compared with controls in two children German cohorts. However, the bronchial hyperreactivity (BHR) was significantly lower in asthmatic homozygous or heterozygous for the CC16*38A allele. In contrast, Candelaria et al.37 reported no association between the A38G polymorphism and rhinitis, atopy, FEV1, FCV, BHR or peripheral blood eosinophils level. Suggesting a role of CC16 in the pathogenesis of asthma independent of atopy. A study on Taiwanese children40 reported a significant association between CC16 G+38A variant and increased risk of asthma, this association directly correlated with the asthma severity. Children with the GA and AA genotypes showed significantly lower serum CC16 and BHR when compared with those with the GG. However, a recently meta-analysis reported no association between the CC16 gene A38G polymorphism and an increased risk of asthma, this result was similar in the analysis stratified by race and age (children or adults).41
There may be several reasons we did not find differences for serum CC16 levels between pre-schoolers with a positive vs. negative API. First, since we studied pre-schoolers and their mean age of asthma/wheezing onset was 12 months of age, the time affected by the disease was perhaps too short to affect the CC16 levels. For example, CC16 decreased only in asthmatic adults with >10 years of disease but not in those with less time.29 Second, in the vast majority of our pre-schoolers, the severity of asthma/wheezing was low to moderate, in contrast with the reported altered levels in severe asthmatic children.28 Third, since it has been shown that CC16*38A might intrinsically influence the CC16 expression, maybe only this polymorphism would be related to increased risk of asthma (∼positive API). Fourth, the absence of difference between positive API (mainly atopy condition) and negative API seems to reflect the similar pattern showed between atopic and non-atopic asthmatic adults,37 and in children with and without parental history of allergy.3 Also, the lack of correlation of CC16 and peripheral blood eosinophils we found, is in agreement with studies carried out in asthmatic adults.37 Because CC16 protein is primarily expressed in the lung, its anti-inflammatory effects are likely to be most pronounced in the airways but not to any other atopy-related phenotype.39 Fifth, CC16 was correlated with a decline in lung function, but most children with asthma have normal lung function.42 Finally, it has been reported that the exposure to tobacco smoke may affect the serum CC16,43 in our group the exposure to tobacco had a similar distribution between groups.
The present study has limitations. First, as a study on pre-schoolers with mild-moderate disease (∼3 wheezing episodes in the previous year) we cannot know if serum CC16 could be different in children with more severe disease and more time of duration of the disease. Second, no control group (pre-schoolers without recurrent wheezing/asthma) was included. Third, the number of participants was small. Fourth, pulmonary function and BHR was not tested: even though they are not easy to perform in pre-schoolers it would be important to establish if serum CC16 level is related with lung function or BHR since CC16 has its main anti-inflammatory effect in the airways.
In conclusion, in contrast to our hypothesis, recurrent wheezing pre-schoolers with positive API (∼allergic or type 2 inflammation asthma) have no different CC16 serum levels than those with negative API. No correlation of serum CC16 levels and peripheral eosinophils blood was found. Larger prospective studies on asthmatic pre-schoolers and the inclusion of a control group need to be carried out to confirm our findings. Studies of the polymorphism of gene controlling CC16 expression in pre-schoolers with asthma risk (e.g. positive API) need to be performed.
FundingDivision of Paediatrics, Pontificia Universidad Catolica de Chile (Grant # 5-14).
Conflict of interestThe authors declare that there are no conflicts of interest.