IL-17-producing B cells can be identified in both mice and human and were named B17 cells. The role of B17 cells still needs to be elucidated and its inflammatory or regulatory functions remain controversial.
ObjectiveWe evaluate the effect of maternal immunization with OVA on offspring B cells that produces IL-17 and can show a regulatory potential by IL-10 production.
MethodsC57BL/6 WT, IL-10−/− or CD28−/− female mice were immunized or not with OVA in Alum, and immunized females were boosted after 10 and 20 days. Immunized and non-immunized females were mated, and pups from both groups were evaluated at 3 or 20 days old (d.o.). Some offspring from the aforementioned two groups were immunized with OVA at 3 d.o., boosted after 10 days and evaluated at 20 d.o.
ResultsMaternal immunization with OVA induced offspring B cells to produce IL-17 at higher intensity compared to the control group of offspring at 3 d.o. This effect was maintained until 20 d.o. and even after neonatal immunization with OVA. The co-production of IL-10 on offspring IL-17+B cells is up-regulated in response to maternal immunization with OVA. Maternal immunization with OVA on IL-10−/− mice reveals reduced percentage and mean of fluorescence intensity of IL-17 on B cells of offspring.
ConclusionPreconception OVA immunization can induce offspring B cells that produce IL-17 at higher intensity and co-produce mainly IL-10. This could be the reason why B17 cells had been described in the literature with controversial roles upon their regulatory function.
Mechanisms by which IgE-mediated allergic hypersensitivity reactions can be regulated in offspring have been investigated by our group in the last 15 years.1–11 In this period, we suggest the role of maternal immunization inhibiting allergy on offspring 12 and we recently demonstrate that IgG can play a pivotal role in this mechanism in mice and humans,13,14 a hypothesis named “MatIgG primary modulation theory”.15 In these observations, B cells seem to be of great importance as mediators of maternal IgG effects and became the main population investigated by our group.
In 2012, it was described that CD19highCD5+ B cells can dominantly produce cytokines, especially IL-17. This IL-17-producing B cells seem to exert a pivotal role for immune tolerance of non-IgE-mediated food allergy in atopic dermatitis patients, and occasionally they were named as Br17 cells.16 One year later, a novel IL-17A-producing B cell population was identified in both mice and humans using an in vitro model of parasite-derived trans-sialidase exposure of primary B cells and were named B17 cells,17 which was subsequently observed in rheumatoid arthritis.18 More recently, a study provided evidence that, in addition to Th17 cells, B cells also participate in dysregulated IL-17A production in response to parasite infection. In this work, the authors suggest that excretory–secretory products of Echinococcus granulosus protoscoleces significantly inhibit the proinflammatory response by direct induction of IL-10-secreting regulatory B cells (B10 cells) and inhibition of B17 and Th17 cells, thereby downregulating anti-parasitic immunity.19 At the moment, the role of B17 cells remains controversial with suggestions of opposite effects on allergic inflammation and parasitic infection.
Proposing to contribute with the elucidation of B17 cells role, we performed an evaluation of this population in a well-standardized murine protocol where the allergic inhibition was described with the induction of the main regulatory population of B cells, B10 cells.
MethodsMiceC57BL/6 inbred wild-type mice (WT) or IL-10 and CD28 genetically deficient (IL-10−/− and CD28−/−) males and females were used at 8–10 weeks old. The animals were purchased through the Central Animal Facility of the School of Medicine and Institute of Biomedical Sciences-USP. The offspring (F1) of both sexes were used. All experiments described in this manuscript were approved by the University of Sao Paulo School of Medicine Animal Ethics Committee (CEP-FMUSP: 122/14-Sao Paulo, SP, Brazil).
Immunization protocolsFemale WT mice were immunized subcutaneously with 1500μg of OVA (Sigma, USA) in 6mg of aluminum hydroxide (FURP, SP, Brazil) and boosted intraperitoneally (ip) after 10 and 20 days with 1000μg of OVA in saline. Females were mated 21 days post-immunization and, as a control group, non-immunized females were mated at the same period. Three-day-old (3 d.o.) offspring were evaluated [Group: 3 d.o.] and divided into two groups. The first were immunized ip with 100μg of OVA in 0.6mg of alum, boosted after 10 days with OVA in saline and evaluated at 20 d.o. [Group: 20 (Im) d.o.]. The second group of animals were not immunized at 3 d.o. and were evaluated at 20 d.o. [Group: 20 d.o.]. Maternal and offspring OVA immunization protocols were also performed using IL-10−/− or CD28−/− mice.
Spleen cell suspensionsSpleens were collected and their cells were isolated for culture or flow cytometry analyses. Single-cell suspensions were prepared with cell strainers (BD Biosciences, MA, USA) and placed in Petri dishes containing RPMI 1640 culture medium (Sigma, USA). The cell suspension was treated with lysis buffer (Biosource-ACK Lysis Buffer, Rockville, MD, USA) for 2min, and the cell suspension was washed twice with RPMI medium. The cells were subsequently resuspended in 1mL of RPMI medium with 10% FBS (III HyClone, Logan, USA), and cellular viability was quantified with 0.5% Trypan blue in a Neubauer chamber.
Flow cytometryFor surface staining, single-cell suspensions were prepared in flow cytometry buffer (PBS, 1% BSA). Directly conjugated antibody with Alexa Fluor 700 anti-CD19 (BD Biosciences, USA) was used at optimal concentrations after the titration experiments. For intracellular cytokines, cells were cultured for 24h at 3×106cells/mL in RPMI 1640 (Gibco, USA) supplemented with 10% heat-inactivated FCS (HyClone, USA) without stimulus in the presence of 10μg/mL brefeldin A (Sigma, USA). Cells were first stained for surface CD19 followed by fixation, permeabilization and intracellular staining with IL-17, IL-4, IFN-γ, IL-10, RORγT or matched isotypes using PBS with 0.5% saponin (Sigma, USA).
Compensation for the instrument was performed using micro beads adsorbed with anti-rat/anti-hamster antibodies (CompBeads-BD Biosciences, USA) and their conjugated antibodies. The acquisition of 100000 events per sample was performed on an LSRFortessa cytometer (BD Biosciences), and analysis was performed using FlowJo software 7.6.5 (Tree Star, USA).
Cell gating was performed using the lymphocyte quadrant (as determined by ratio size/granularity) and CD19+ cells (to determine B cells) based on specific isotype control values as well as the fluorescence minus one (FMO) to determine intracellular cytokine levels. All analyses were performed using FlowJo software (Tree Star, USA).
Intracellular cytokine detection was previously standardized using as a positive control the stimulus with phorbol 12-myristate 13-acetate (PMA) and ionomycin, and as negative control we used ex vivo stained cells.14,20
Purification of IgGIgG antibodies were purified from the sera of mice that were immunized with OVA (40 days after immunization) or from non-immunized mice using a Melon Gel IgG Spin Purification kit, as previously described by our group.13,14 Purified IgGs were sterilized using 0.20-micron filters (Corning, Germany), and their IgG concentrations were determined with the Coomassie Protein Assay Reagent (Pierce, USA) according to the manufacturer's instructions.
Cell cultureTo investigate the in vitro effect of purified maternal antibodies on offspring B cells, splenocytes from 3 d.o. offspring of immunized or non-immunized mothers were cultured for 120h at a density of 3×106cells/mL in RPMI 1640 (Gibco, USA) supplemented with 10% heat-inactivated FCS (HyClone, USA) in the presence of 100μg/mL of OVA with 100μg/mL purified IgG from immunized or non-immunized mothers. Some culture wells were previously blocked with 10μg/mL purified anti-CD16/32 (2.4G2). For the evaluation of intracellular cytokine production and RORγT expression, all of the culture wells were given 10mg/mL brefeldin A (Sigma, USA) 24h before flow cytometric evaluation.
Statistical analysis and number of samplesStatistical analysis was performed with GraphPad Prism 5.0 (GraphPad Software Inc., La Jolla, CA, USA). All the data in this paper were taken from three to five separate experiments with 9–12 mice per group. Differences were considered significant when p<0.5 as assessed by the Mann–Whitney test.
ResultsMaternal immunization can induce IL-17 production by offspring B cells that co-produces IL-10.
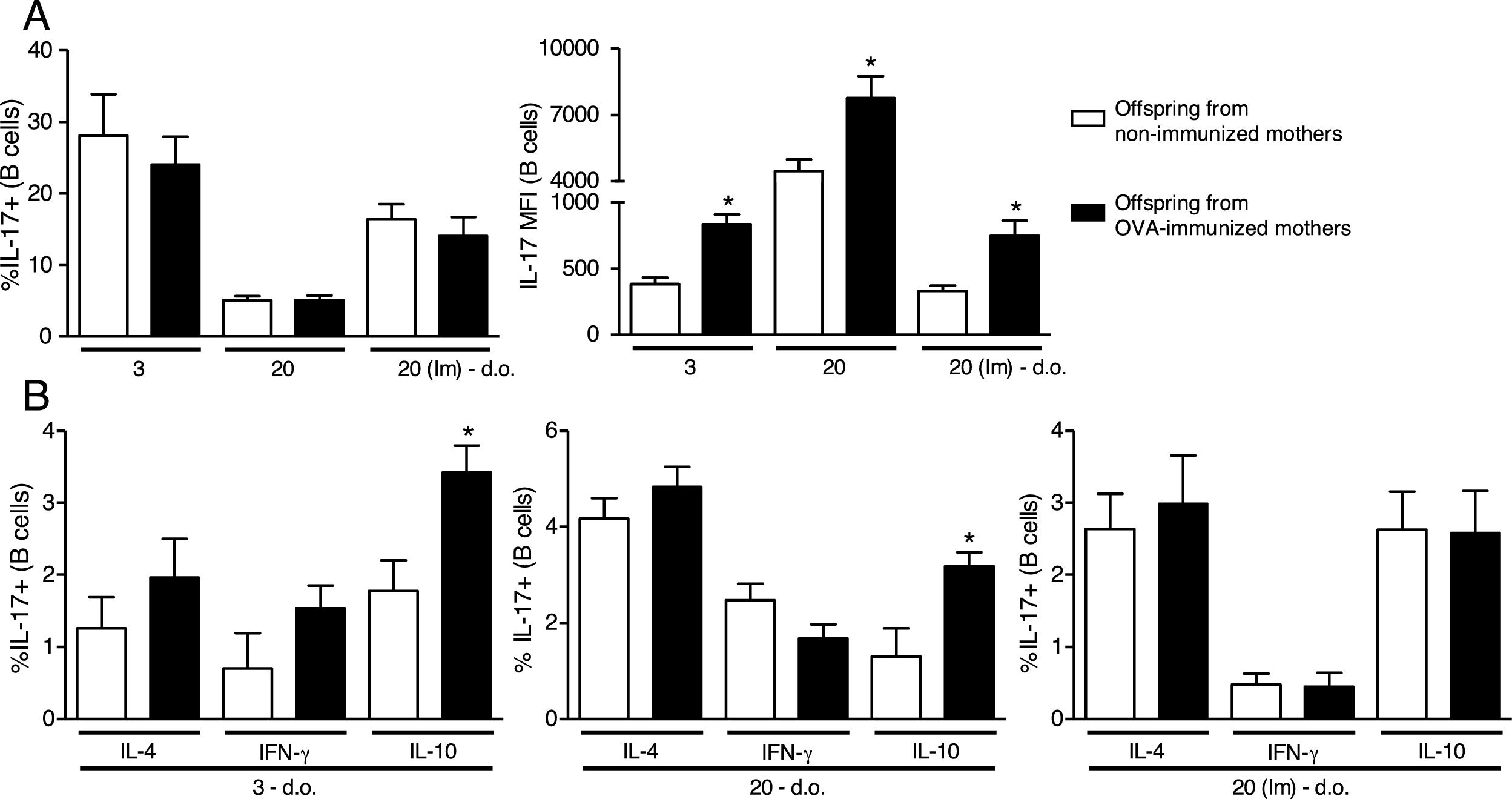
Analyzing a standardized maternal immunization protocol with OVA that inhibits offspring allergy development,1,8,14 we could observe that maternal immunization in WT mice did not influence intracellular IL-17 percentage on offspring B cells at any period evaluated (Fig. 1A). However, the evaluation of intracellular IL-17 intensity by the MFI reveals that maternal immunization could induce higher levels of IL-17 on offspring B cells compared to the respective control group of offspring at 3 d.o. which was maintained until 20 d.o. and even after offspring OVA immunization at the neonatal period (Fig. 1A).
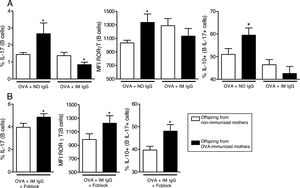
Effect of maternal immunization on offspring B cells IL-17 production and IL-17+cells co-production of IL-4, IFN-γ and IL-10. Offspring from immunized or non-immunized WT mothers were evaluated at 3 (3 d.o.—n=11 on each group) or 20 (20 d.o.—n=10 on each group) days old or immunized with OVA in the neonatal period and evaluated at 20 days old [20 (Im) d.o.—n=9 on each group]. Splenic B cell (CD19+) intracellular IL-17 percentage and mean of fluorescence intensity (MFI) was evaluated by flow cytometry (A). The same groups were evaluated for IL-4, IFN-γ and IL-10 co-production on IL-17+B cells by flow cytometry (B). Bars represent the mean±standard error. *P≤0.05 compared with the offspring of the respective non-immunized mothers.
Next, we evaluated the co-production of IL-4, IFN-γ and IL-10 on offspring IL-17+B cells, and we could observe that maternal immunization with OVA induced an up-regulation of IL-10 co-production on offspring IL-17+B cells at 3 and 20 d.o., an effect that was not observed after neonatal immunization (Fig. 1B). Offspring co-production of IL-4 and IFN-γ by IL-17+B cells was not influenced by maternal immunization at any period evaluated (Fig. 1B).
The absence of IL-10 can block IL-17 induction on offspring B cells but the absence of B/T cells collaboration does not.
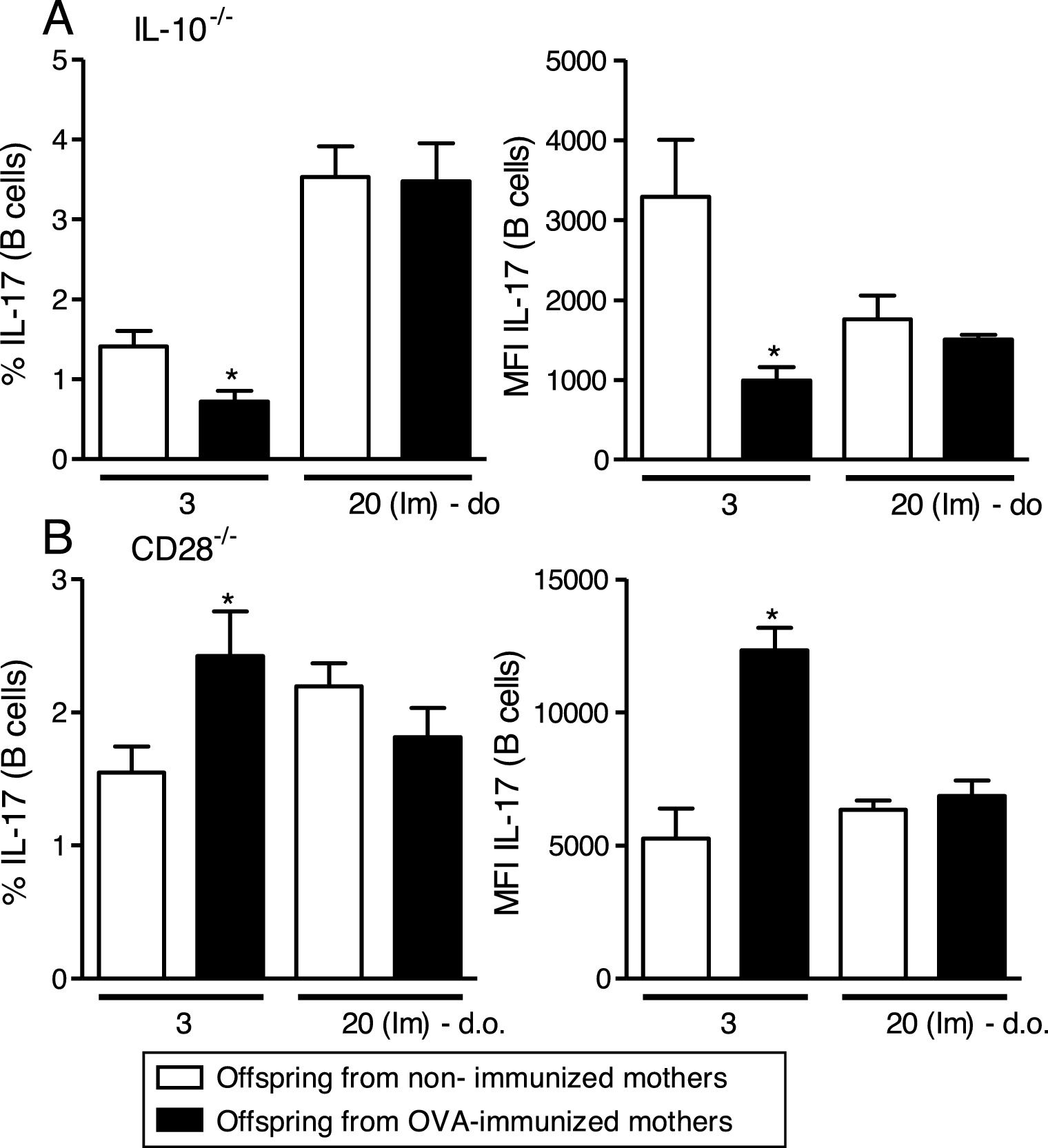
Maternal immunization with OVA on IL-10−/− mice reveals reduced percentage and MFI of IL-17 on offspring B cells at 3 d.o. compared to the control group, an effect that was not observed after neonatal immunization (Fig. 2A). An opposite effect was observed after maternal immunization with OVA on CD28−/− mice, with augmented percentage and MFI of IL-17 on offspring B cells at 3 d.o. compared to the control group, but similar to IL-10−/−, no influence was observed after neonatal immunization (Fig. 2B).
Effect of IL-10 or CD28 blockage upon IL-17 induction on offspring B cells.
Offspring from immunized or non-immunized IL-10−/− or CD28−/− mothers were evaluated at 3 (3 d.o.—n=8–9 on each group) days old or immunized with OVA in the neonatal period and evaluated at 20 days old [20 (Im) d.o.—n=8–9 on each group]. Splenic B cell (CD19+) intracellular IL-17 percentage and mean of fluorescence intensity (MFI) was evaluated by flow cytometry on IL-10−/− (A) or CD28−/− (B) pups. Bars represent the mean±standard error. *P≤0.05 compared with the offspring of the respective non-immunized mothers.
The induction of IL-17 on offspring B cells is antigen-specific and can be blocked by specific maternal IgG.
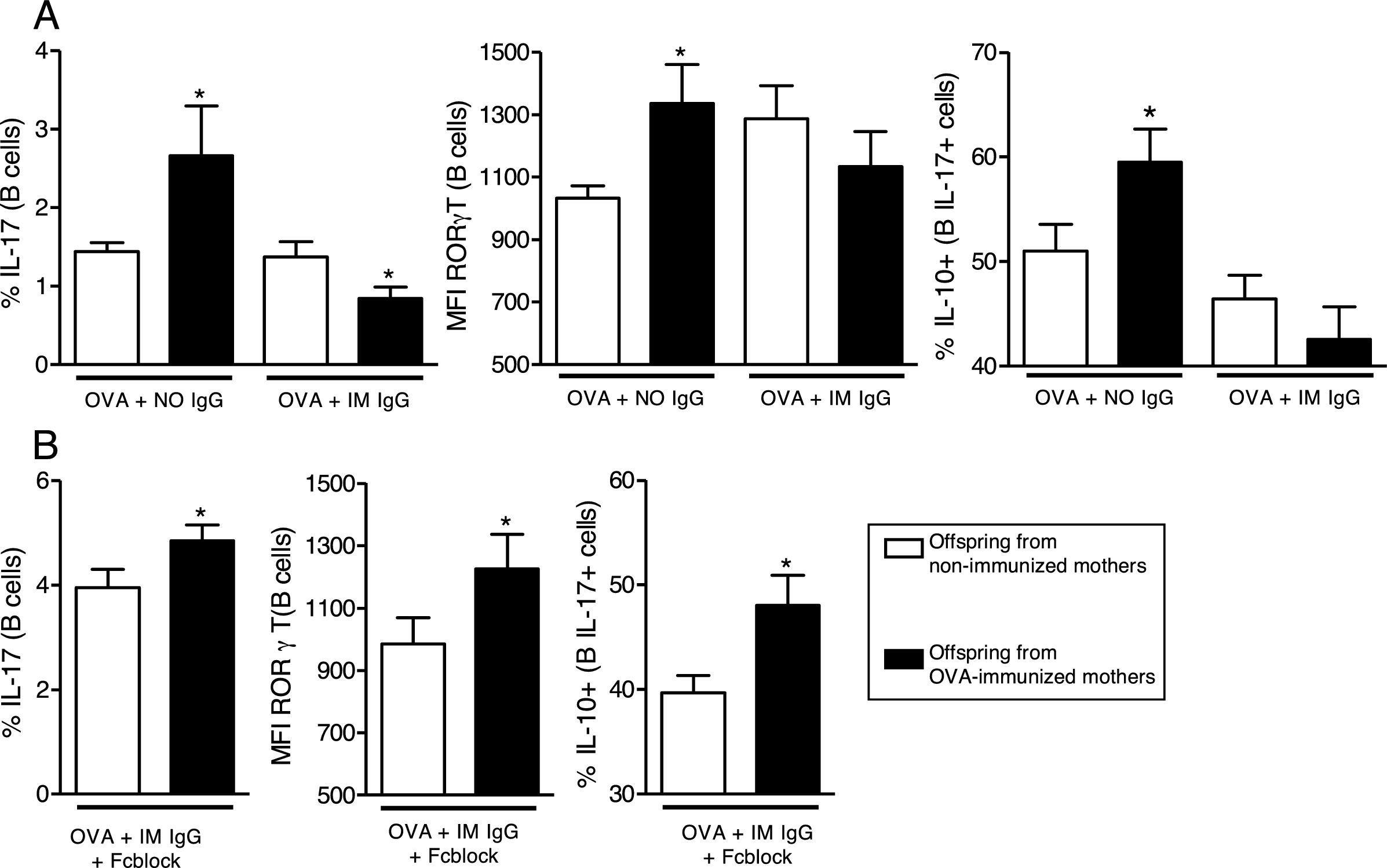
We then evaluated the effects of in vitro OVA stimulation in the presence of purified maternal IgG on neonatal offspring splenocytes. In the presence of OVA and IgG from non-immunized mothers, offspring from immunized mothers revealed augmented levels of IL-17 and RORγT on B cells and augmented co-production of IL-10 on IL-17+B cells (Fig. 3A). This effect could not be observed in the presence of IgG from immunized mothers (Fig. 3A). To evaluate the involvement of IgG receptors, we repeated the experiments using OVA and IgG from immunized mothers after IgG receptors blockage; under this condition, the results became equal to those observed in the OVA/IgG from non-immune mothers condition with augmented levels of IL-17 and RORγT on B cells and augmented co-production of IL-10 on IL-17+B cells of offspring from immunized mothers (Fig. 3B).
In vitro induction of IL-17 and the effect of maternal IgG. Splenocytes of offspring derived from immunized or non-immunized mothers were cultured (n=10 on each group) for 7 days with 20μg/mL OVA and 100μg/mL purified IgG from non-immunized (NO IgG) or immunized mothers (IM IgG) and the percentage of intracellular IL-17, RORγT or co-production of IL-17 and IL-10 of offspring B cells was evaluated by flow cytometry (A). We also evaluated the same flow cytometry parameters adding 10μg/mL of FcγRII/III-blocking Ab in cultures with OVA and IM IgG (B). Bars represent the mean±standard error. *P≤0.05 compared with the offspring of the respective non-immunized mothers.
In the present work, we evaluate an additional parameter in a well-standardized preconceptional OVA immunization protocol that has been described by our group. This protocol can inhibit offspring lung allergic inflammation8 and induce offspring B regulatory cells that produce IL-10 (B10 cells) that were mainly induced by maternal IgG.14 Since B17 cells were recently described as CD19+CD1dhigh B cells,19 a phenotype related to B10 cells, we decided to evaluate this population in our murine model.
In our model, the frequency of B17 cells was similar among offspring from both groups, but in those from immune mothers, these cells spontaneously produce IL-17 in a higher intensity, suggesting a high potential to produce IL-17.
The evaluation of IL-17 production by B cells is very scarce in the literature, and some evidence reveals that B10 cells are related to the regulation of IL-17 production but only by TCD4 cells (Th17). This has already been demonstrated in murine models of arthritis and in human diseases as psoriasis or systemic sclerosis.21–24 Since our model is characterized by the induction of B10 cells, we evaluate whether these B17 cells could be related to IL-10 co-production that could only be observed in those groups producing IL-17 at high intensity. Since its characterization, the co-production of other cytokines was not evaluated on B17 cells, and this observation is unprecedented and can justify the controversial role of B17 cells in the literature suggesting inflammatory and regulatory roles and also the description of a B10-like phenotype of B17 cells.16,17,19
In the present work, we did not evaluate molecular parameters that can represent a link between IL-10 and IL-17 production on B cells or the importance of T cells collaborations in this mechanism. However, the use of IL-10−/− mice reveals that B17 population frequency and IL-17 intensity of production are committed in the absence of IL-10. Furthermore, the use of CD28−/− mice reveals that B17 population can be induced in the absence of an effective B/T cells collaboration.
We also evidenced in vitro that B17 cells produce IL-17 in response to OVA which can be avoided by allergen-specific IgG via IgG receptors interaction. Emphasizing differences between B17 and B10 cells, this last observation is very important, while B10 cells do respond to allergen but also to specific IgG in the absence of allergen and the blockage of IgG receptors results in augmented IL-10 production,14 B17 cells seem to respond in the presence of OVA and are inhibited in the presence of specific IgG.
Taken together, our observations suggest that B17 and B10 can share the similarity to produce IL-10, at least partially, which can mislead some experimental evidence about these populations. Moreover, B17 cells seem to respond in the presence of allergen-producing IL-17 what can be corroborated with a pro-inflammatory function and is not in accordance with B10 cells functions. In conclusion, B17 coproduction of IL-10 and its IL-10 dependency could be the reasons why this cell population had been described with controversial roles upon its function.
Sources of fundingThis study was funded by grants #2015/17256-3, #2017/18558-9 and #2013/22820-0 from the São Paulo Research Foundation (FAPESP) and by grant #115603/2015-8 from the National Council for Scientific and Technological Development (CNPq).
Conflicts of interestThe authors declare that they have no relevant conflicts of interest.




![Effect of maternal immunization on offspring B cells IL-17 production and IL-17+cells co-production of IL-4, IFN-γ and IL-10. Offspring from immunized or non-immunized WT mothers were evaluated at 3 (3 d.o.—n=11 on each group) or 20 (20 d.o.—n=10 on each group) days old or immunized with OVA in the neonatal period and evaluated at 20 days old [20 (Im) d.o.—n=9 on each group]. Splenic B cell (CD19+) intracellular IL-17 percentage and mean of fluorescence intensity (MFI) was evaluated by flow cytometry (A). The same groups were evaluated for IL-4, IFN-γ and IL-10 co-production on IL-17+B cells by flow cytometry (B). Bars represent the mean±standard error. *P≤0.05 compared with the offspring of the respective non-immunized mothers. Effect of maternal immunization on offspring B cells IL-17 production and IL-17+cells co-production of IL-4, IFN-γ and IL-10. Offspring from immunized or non-immunized WT mothers were evaluated at 3 (3 d.o.—n=11 on each group) or 20 (20 d.o.—n=10 on each group) days old or immunized with OVA in the neonatal period and evaluated at 20 days old [20 (Im) d.o.—n=9 on each group]. Splenic B cell (CD19+) intracellular IL-17 percentage and mean of fluorescence intensity (MFI) was evaluated by flow cytometry (A). The same groups were evaluated for IL-4, IFN-γ and IL-10 co-production on IL-17+B cells by flow cytometry (B). Bars represent the mean±standard error. *P≤0.05 compared with the offspring of the respective non-immunized mothers.](https://static.elsevier.es/multimedia/03010546/0000004600000005/v1_201808030437/S0301054618300697/v1_201808030437/en/main.assets/thumbnail/gr1.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)
![Effect of IL-10 or CD28 blockage upon IL-17 induction on offspring B cells. Offspring from immunized or non-immunized IL-10−/− or CD28−/− mothers were evaluated at 3 (3 d.o.—n=8–9 on each group) days old or immunized with OVA in the neonatal period and evaluated at 20 days old [20 (Im) d.o.—n=8–9 on each group]. Splenic B cell (CD19+) intracellular IL-17 percentage and mean of fluorescence intensity (MFI) was evaluated by flow cytometry on IL-10−/− (A) or CD28−/− (B) pups. Bars represent the mean±standard error. *P≤0.05 compared with the offspring of the respective non-immunized mothers. Effect of IL-10 or CD28 blockage upon IL-17 induction on offspring B cells. Offspring from immunized or non-immunized IL-10−/− or CD28−/− mothers were evaluated at 3 (3 d.o.—n=8–9 on each group) days old or immunized with OVA in the neonatal period and evaluated at 20 days old [20 (Im) d.o.—n=8–9 on each group]. Splenic B cell (CD19+) intracellular IL-17 percentage and mean of fluorescence intensity (MFI) was evaluated by flow cytometry on IL-10−/− (A) or CD28−/− (B) pups. Bars represent the mean±standard error. *P≤0.05 compared with the offspring of the respective non-immunized mothers.](https://static.elsevier.es/multimedia/03010546/0000004600000005/v1_201808030437/S0301054618300697/v1_201808030437/en/main.assets/thumbnail/gr2.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)