Transplantation-acquired food allergies (TAFA) are frequently reported and considered to be caused by immunosuppressive therapy.
The aim of this study was to investigate the allergic and immunologic responses in children who had liver or kidney transplantations.
MethodsTwelve children receiving liver transplantations and 10 children receiving kidney transplantations were investigated. All children underwent the allergy work-up and in most of them, lymphocyte screening and serum cytokine measurements were also performed.
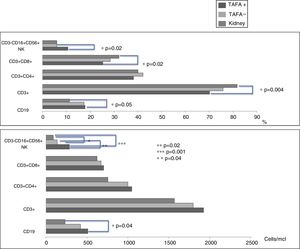
ResultsTAFA were found in 7/12 (58%) children with liver transplantations and in none of the 10 children with kidney transplantations. The mean age at transplantation was significantly lower in children who underwent liver transplantations (p<0.001). The immunosuppressive therapy administered to children with liver transplantation was tacrolimus in 11 patients and cyclosporine in one patient, while all 10 children with kidney transplantation received tacrolimus plus mycophenolate. The most common antigenic food was egg. The natural killer (NK) cell numbers were significantly higher in liver-transplant children than in kidney-transplant children. No significant differences were found in the serum cytokine levels.
ConclusionsThis study confirms that liver-transplant children treated with tacrolimus alone have a higher risk of developing TAFA than kidney-transplant children treated with tacrolimus plus mycophenolate. NK cells might be involved in this difference.
Food allergies after transplantation are frequently reported in literature as transplantation-acquired food allergy (TAFA). The reported prevalence of TAFA in paediatric patients is between 6% and 57%.1 Tacrolimus-based maintenance immunosuppression, frequently used in paediatric solid-organ transplantation, seems to play a role by inducing a shift towards T-helper 2 cells.1,2
The occurrence of new-onset food allergy has increasingly been reported after paediatric liver transplantation. In addition, new-onset food allergy appears to be more common after liver transplantation compared with other solid-organ transplantation in patients who receive similar immunosuppressive therapy.3–5
This finding suggests that tacrolimus is not the only predisposing factor in the development of food allergy. To date, the underlying physio-pathological mechanism has not been fully understood,6 but a T-cell imbalance is considered the most probable effect.7
In this study, we investigated allergic and immunologic responses in children who had undergone liver or kidney transplantations.
MethodsPatientsFrom 1992 to 2012, we enrolled 22 patients (12 liver-transplant and 10 kidney-transplant) who were followed at the Anna Meyer Children's University Hospital.
Informed consent was given by the parents and/or patients, and after obtaining written approval, the data were collected from the medical records, including the following items: sex, age, indication for transplantation, age at the time of transplantation, and immunosuppressive drugs administered. For each patient, we also collected the clinical history related to any allergic reactions occurring before or after the transplantation, focusing on the presence of atopy in first-degree relatives as well. In patients who had immediate cutaneous, respiratory, and/or gastrointestinal symptoms, or anaphylactic reactions suspected of deriving from food allergy, a detailed medical history was obtained in order to identify potential allergens.
The diagnosis of food allergy was based on the presence of a positive skin prick test (SPT) or specific IgE (s-IgE), and convincing symptoms following specific food exposure. In some doubtful cases (negative SPT and/or s-IgE, but a positive clinical history), an oral provocation test was also performed. Symptoms were considered severe in case of anaphylactic reactions, and persistent if recurrent during the previous six months.
During the period the children suffered from allergy, lymphocyte screening was performed, and serum cytokine levels were determined in most of the patients.
Skin prick testsSPTs were performed and read after 10–15min by the same investigator to avoid operator-related variability. We tested all children with commercial extracts at a 0.1mg/mL concentration (Alk Abellò, Milan, Italy) of common inhalants (pollens, mites, moulds, cat and dog epithelia, grass, olive, Cupressus arizonica, Betula pendula, Artemisia vulgaris, Carpinus betulus, and Parietaria mix) and foods (milk, albumen, soy, wheat, cod fish, peanut). Patients suspected of being allergic to other specific foods were tested with the culprit food using the skin prick or prick-to-prick methods. Skin prick tests were performed on the volar surface of the forearm using a standard 1-mm tip lancet, according to the recommendations of the European Academy of Allergy and Clinical Immunology. Positive controls for the prick and prick-to-prick tests were performed with histamine (Alk-Abellò, Milan, Italy: 10mg/mL concentration). Normal saline was used as a negative control for the prick and prick-to-prick tests. The SPT results were considered positive if the difference between the mean diameter of the wheal and the negative control was at least 3mm. The children had been off antihistamines and oral corticosteroids for 10 days before the skin testing.
In vitro testsSerum IgE detectionTotal IgE levels were detected in most of the children enrolled (12 liver-transplant and six kidney-transplant patients) using a radioimmunosorbent test (kU/L), and s-IgE to the antigenic food were measured in all children with a history of immediate reactions (Immunocap RAST, Uppsala, Sweden). A positive result was obtained if the level of s-IgE to the food was >0.35kU/L.
Cell analysisUsing flow cytometry (BD Multitest™ 6-colour TBNK), we measured the CD4/CD8 ratios and the numbers and percentages of the following peripheral cell types in 12 liver-transplant and seven kidney-transplant patients: T cells (CD3+), B cells (CD19+), T helper lymphocytes (CD3+CD4+), cytotoxic T lymphocytes (CD3+CD8+), and natural killer (NK) cells (CD3−CD16+CD56+).
Cytokine detectionSerum cytokine levels (pg/mL) were measured in nine liver-transplant children, seven kidney-transplant children and eight healthy controls (seven females and one male; mean age: 97 months [20–228 months]). The cytokines detected were IL-2, IL-4, IL-6, IL-10, TNF, IFN-γ, and IL-17A (BD™ Cytometric Bead Array).
Statistical analysisThe frequencies, percentages, and means were calculated using descriptive statistics. The means were compared using the Student's t-test, and the frequencies were compared using a χ2 test. A p-value of 0.05 or less was considered statistically significant.
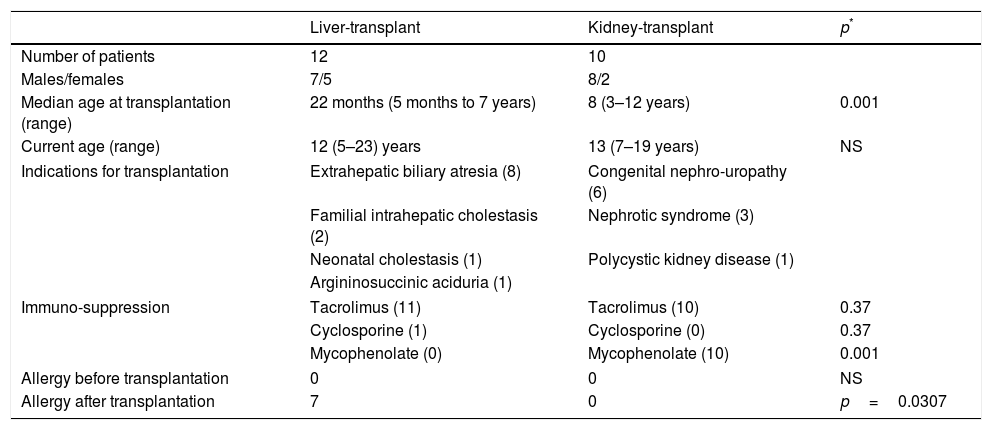
ResultsTAFA was found in 7/12 (58%) children who had liver transplantations and in none of the 10 children who had kidney transplantations (p=0.0307; OR 28.6; 95% CI: 1.4–600.4). The mean age at transplantation was significantly lower in the children who underwent liver transplantations (p<0.001) (Table 1).
Characteristics of liver- and kidney-transplant patients.
| Liver-transplant | Kidney-transplant | p* | |
|---|---|---|---|
| Number of patients | 12 | 10 | |
| Males/females | 7/5 | 8/2 | |
| Median age at transplantation (range) | 22 months (5 months to 7 years) | 8 (3–12 years) | 0.001 |
| Current age (range) | 12 (5–23) years | 13 (7–19 years) | NS |
| Indications for transplantation | Extrahepatic biliary atresia (8) | Congenital nephro-uropathy (6) | |
| Familial intrahepatic cholestasis (2) | Nephrotic syndrome (3) | ||
| Neonatal cholestasis (1) | Polycystic kidney disease (1) | ||
| Argininosuccinic aciduria (1) | |||
| Immuno-suppression | Tacrolimus (11) | Tacrolimus (10) | 0.37 |
| Cyclosporine (1) | Cyclosporine (0) | 0.37 | |
| Mycophenolate (0) | Mycophenolate (10) | 0.001 | |
| Allergy before transplantation | 0 | 0 | NS |
| Allergy after transplantation | 7 | 0 | p=0.0307 |
The immunosuppressive therapy administered to children with liver transplantation was tacrolimus in 11 patients and cyclosporine in one patient, while all 10 children with kidney transplantation received tacrolimus plus mycophenolate (p<0.001) (Table 1).
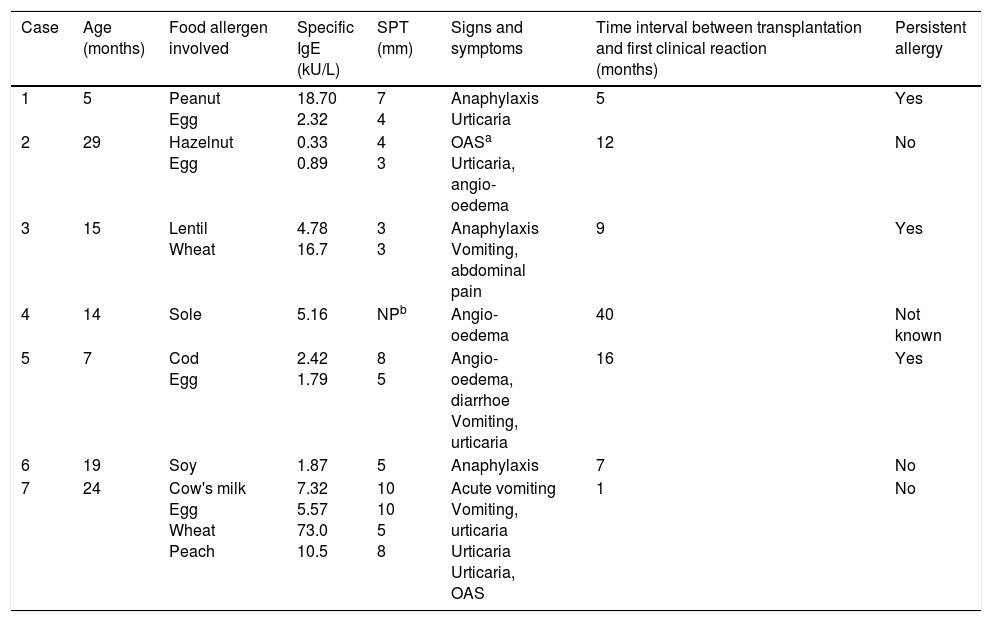
Egg was the most common antigenic food. Three out of seven children had severe symptoms (i.e., anaphylaxis), and three out of seven had a persistent allergic manifestation for several years (Table 2).
Clinical and allergic features in patients with post-liver transplantation food allergy.
| Case | Age (months) | Food allergen involved | Specific IgE (kU/L) | SPT (mm) | Signs and symptoms | Time interval between transplantation and first clinical reaction (months) | Persistent allergy |
|---|---|---|---|---|---|---|---|
| 1 | 5 | Peanut Egg | 18.70 2.32 | 7 4 | Anaphylaxis Urticaria | 5 | Yes |
| 2 | 29 | Hazelnut Egg | 0.33 0.89 | 4 3 | OASa Urticaria, angio-oedema | 12 | No |
| 3 | 15 | Lentil Wheat | 4.78 16.7 | 3 3 | Anaphylaxis Vomiting, abdominal pain | 9 | Yes |
| 4 | 14 | Sole | 5.16 | NPb | Angio-oedema | 40 | Not known |
| 5 | 7 | Cod Egg | 2.42 1.79 | 8 5 | Angio-oedema, diarrhoe Vomiting, urticaria | 16 | Yes |
| 6 | 19 | Soy | 1.87 | 5 | Anaphylaxis | 7 | No |
| 7 | 24 | Cow's milk Egg Wheat Peach | 7.32 5.57 73.0 10.5 | 10 10 5 8 | Acute vomiting Vomiting, urticaria Urticaria Urticaria, OAS | 1 | No |
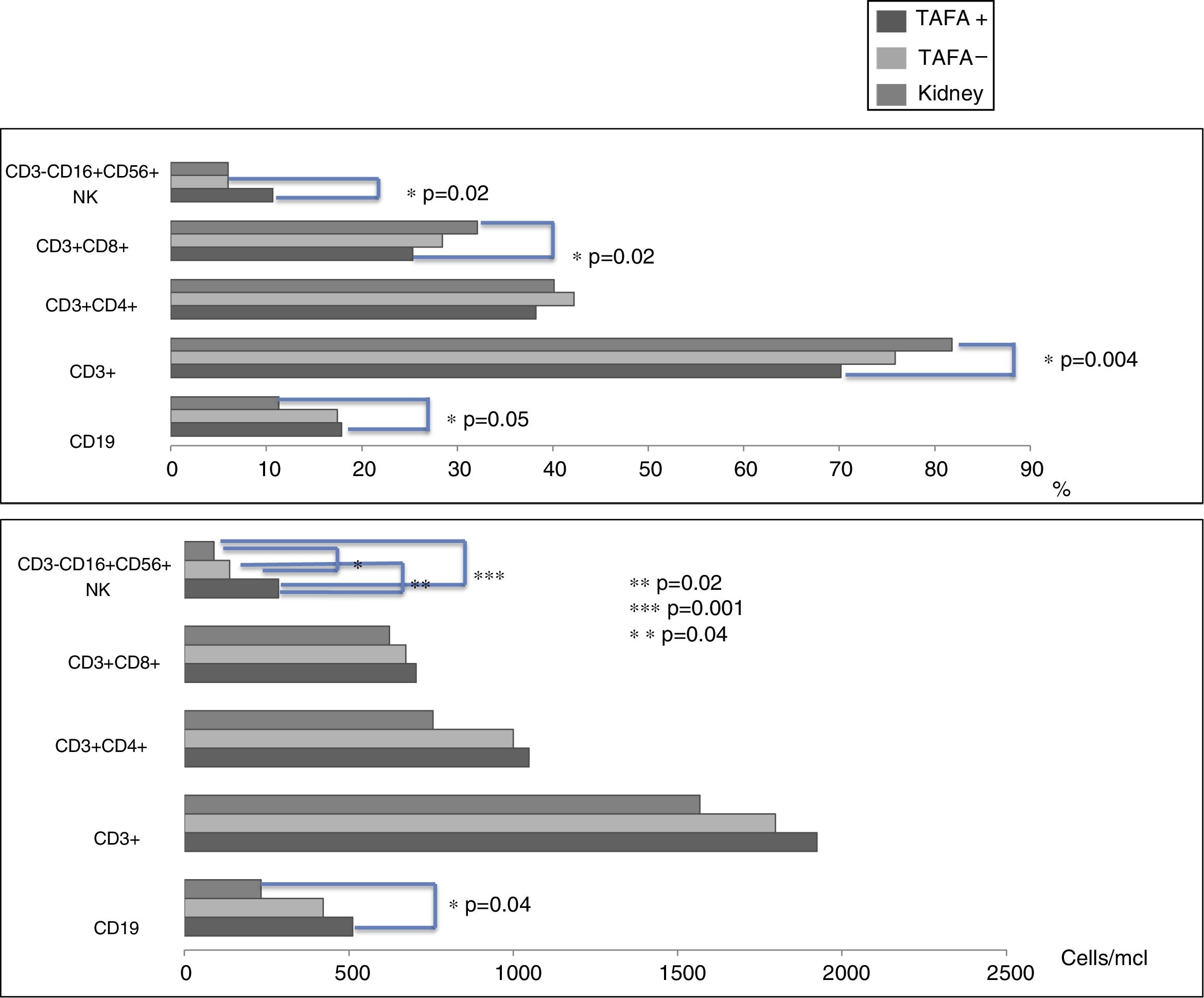
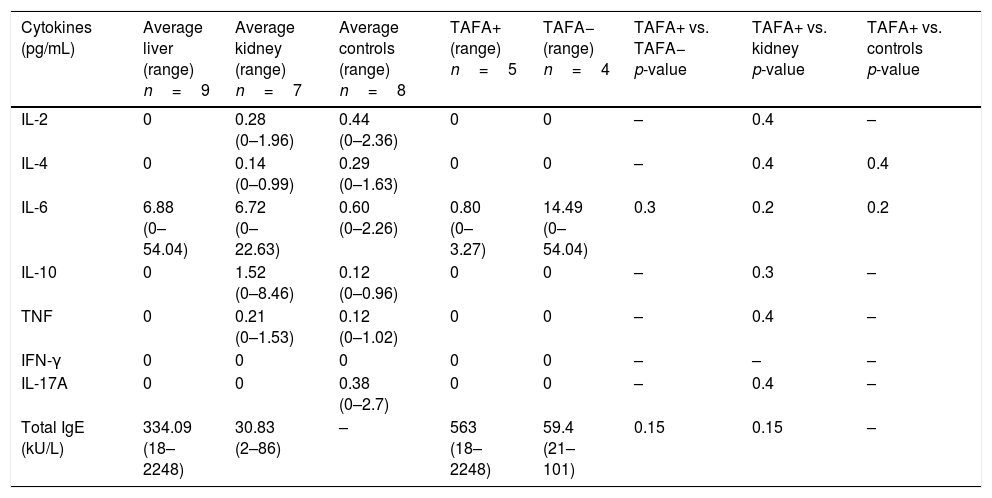
CD3−CD16+CD56+ (NK) cell values (absolute numbers) were significantly higher in children who had liver transplantations than in children who had kidney transplantations, above all in those with liver TAFA, rather than those without liver TAFA (both absolute numbers and percentages) (Fig. 1). The CD4/CD8 ratio was calculated in the TAFA positive-patient group [mean: 1.6; range: 0.9–2.7], in the TAFA negative-patient group [mean: 1.8; range: 0.6–3.7] and in the kidney-transplant group [mean: 1.3; range: 0.8–1.8]. No significant differences were observed between the CD4/CD8 values of the three groups (data not shown). The levels of IL-6 were detectable in both liver- and kidney-transplant children, with an order of magnitude higher than the IL-6 levels in the control group. Similarly, IL-10 also showed a higher order of magnitude in the kidney-transplant group than in the control group. However, no significant differences were found in the serum cytokine levels in children who had liver transplantations compared with children with kidney transplantations (Table 3).
Serum cytokines in liver-transplant patients with and without TAFA and kidney-transplant patients and controls; total IgE in liver- and kidney-transplant patients and in allergic and non-allergic liver-transplant patients.
| Cytokines (pg/mL) | Average liver (range) n=9 | Average kidney (range) n=7 | Average controls (range) n=8 | TAFA+ (range) n=5 | TAFA− (range) n=4 | TAFA+ vs. TAFA− p-value | TAFA+ vs. kidney p-value | TAFA+ vs. controls p-value |
|---|---|---|---|---|---|---|---|---|
| IL-2 | 0 | 0.28 (0–1.96) | 0.44 (0–2.36) | 0 | 0 | – | 0.4 | – |
| IL-4 | 0 | 0.14 (0–0.99) | 0.29 (0–1.63) | 0 | 0 | – | 0.4 | 0.4 |
| IL-6 | 6.88 (0–54.04) | 6.72 (0–22.63) | 0.60 (0–2.26) | 0.80 (0–3.27) | 14.49 (0–54.04) | 0.3 | 0.2 | 0.2 |
| IL-10 | 0 | 1.52 (0–8.46) | 0.12 (0–0.96) | 0 | 0 | – | 0.3 | – |
| TNF | 0 | 0.21 (0–1.53) | 0.12 (0–1.02) | 0 | 0 | – | 0.4 | – |
| IFN-γ | 0 | 0 | 0 | 0 | 0 | – | – | – |
| IL-17A | 0 | 0 | 0.38 (0–2.7) | 0 | 0 | – | 0.4 | – |
| Total IgE (kU/L) | 334.09 (18–2248) | 30.83 (2–86) | – | 563 (18–2248) | 59.4 (21–101) | 0.15 | 0.15 | – |
TAFA: transplantation-acquired food allergy.
TAFA is a known side effect of solid organ transplantation. However, the incidence, clinical manifestation, natural history and pathogenesis of TAFA are not fully understood. Previous studies have suggested that TAFA is rare, mild and transient8 and that it preferentially occurs in patients receiving liver transplantations, young patients and patients receiving tacrolimus immunosuppression. Our study confirmed that TAFA is a complication in children undergoing liver transplantations. However, in contrast to previous studies,8 in our population, it was a very common side effect as 7/12 (58%) of the liver transplantation recipients developed food allergies which were severe and/or persistent in most cases (Table 2). Indeed, three of the children had anaphylaxis and three had persistent allergic manifestations for several years (Table 2), suggesting the need for early diagnosis and prolonged follow-up.
This study shows that after liver transplantation children under tacrolimus treatment have a higher risk of developing new-onset food allergy compared with children who underwent kidney transplantation and received a combined tacrolimus and mycophenolate treatment, as 7/12 of the liver transplantation recipients, but none of the kidney transplantation recipients, developed TAFA.
Moreover, our study confirms that children undergoing liver transplantation were significantly younger than children who had kidney transplantation (Table 1). Indeed, literature suggests that children with TAFA are typically young.9 In one study, those of a younger age at the time of liver transplantation displayed statistically significant differences in the development of allergy.10 Proposed hypotheses for the role of age include the immaturity of oral tolerance, a lack of protective factors (IgA or suppressive CD8+ lymphocytes), feeding with an artificial formula, a poorly diversified diet until liver transplantation, and limited exposure to dietary allergens prior to transplantation, which are likely to induce a highly altered microbial ecology that can affect the function of the regional immune system.11,12
This comparison, by itself, does not support the claim that the risk of developing TAFA is age-dependent, as liver-transplant children are at a higher risk of TAFA than kidney-transplant children, irrespective of age (Table 2; TAFA positive vs. TAFA negative p=0.2).
Most of the children with liver TAFA are allergic to multiple foods, suggesting the need for early diagnosis and accurate follow-up. In one study, the mean duration of food allergy was 16 months,8 but in our study three patients were still symptomatic after many years (Table 2).
An increased prevalence of food allergy was noted specifically in children receiving tacrolimus, but not cyclosporine, immunosuppression.13,14 Our study also suggests that tacrolimus could play a major role in TAFA development as almost all children with liver transplantation received tacrolimus. However, almost the entire liver-transplant group was on tacrolimus (11 out of 12), consequently to confirm this hypothesis it will be compared with additional patients who underwent liver transplantation but received treatment other than tacrolimus.
Normally, T cell-mediated immune responses to dietary antigens are suppressed by means of the clonal deletion of antigen-specific CD4+ T cells through apoptosis, cytokine-mediated active suppression, or both.14 On the other hand, patients with specific IgE-mediated food allergy have T cells that are activated and produce IL-4/IL-13 upon allergen stimulation.15,16 It is increasingly recognised that this type of response is most likely driven by Th2 cytokines secreted by innate cells, such as basophils, iNKt and NK cells, in genetically predisposed individuals and in the presence of food allergy-favouring factors, such as epithelial damage and infection danger signals.17,18
Other immunosuppressive agents used in transplantation conditioning and continuative treatments may also play a significant role in immunomodulation and consequent TAFA development. Indeed, a decrease in food s-IgE levels was recently reported in paediatric patients with liver TAFA after the addition of mycophenolate to tacrolimus.19
Interestingly, different immune suppressants have different effects on NK function, with a stronger inhibition of NK cells if mycophenolate is added to tacrolimus.20 Therefore, we can speculate that a stronger inhibition of NK cells with the consequent abolition of Th1/Th2 secretion would not favour food allergy development. Mycophenolate circulates more in the GI system; therefore it possibly blocks not only Th1 but also Th2 cytokines in the GI tract and liver, which is the most likely site of food allergy sensitisation.20
In our study, no significant modifications of serum cytokines were observed in liver-transplant compared with kidney-transplant children. In the TAFA patients and controls, most of all cytokines were undetectable. This finding is not surprising given the immunosuppressant immunotherapy the patients received and the fact that the sensitisation most likely happened at the tissue level.
In conclusion, our study suggests that young patients undergoing liver transplantation and maintained with tacrolimus alone as an immunosuppressant are at risk of developing TAFA. Furthermore, for the first time, we showed that in these children, NK cells might play a role in developing TAFA. We also showed that precautions should be taken in children undergoing liver transplantation, as in most cases the food allergies were persistent and/or severe. Mycophenolate could be considered in the immunosuppressive regimen due to its anti-allergic effects.
Conflict of interestThe authors have no conflict of interest to declare.