Exhaled breath condensate (EBC) is a completely non-invasive method for the collection of airway secretions to measure intense inflammation in the airways of asthmatics. It has been shown that the childhood asthma control test (c-ACT) is a good tool for use in the evaluation of asthmatics. Whether the c-ACT score and asthma control level correlate with the airway inflammation is not well known. We aimed to evaluate the relationship between exhaled cysteinyl leukotrienes (Cys-LTs) and 8-isoprostane levels and asthma severity, asthma control level and c-ACT score in asthmatic children.
MethodsThirty asthmatic children were evaluated with c-ACT score and pulmonary function tests. Asthma severity and asthma control level were assessed according to GINA. EBC was collected and Cys-LTs and 8-isoprostane concentrations were determined using a specific immunoassay kit.
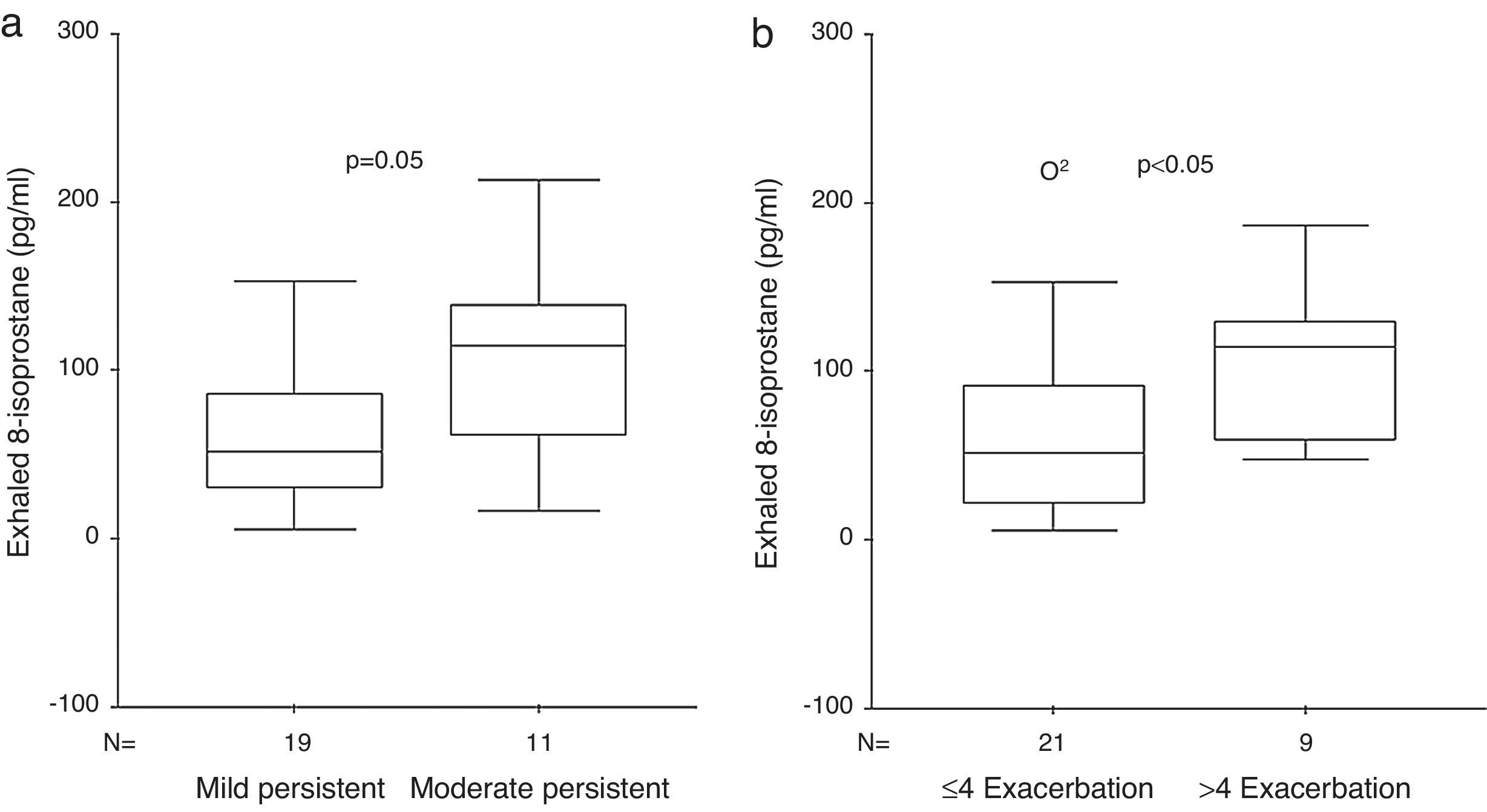
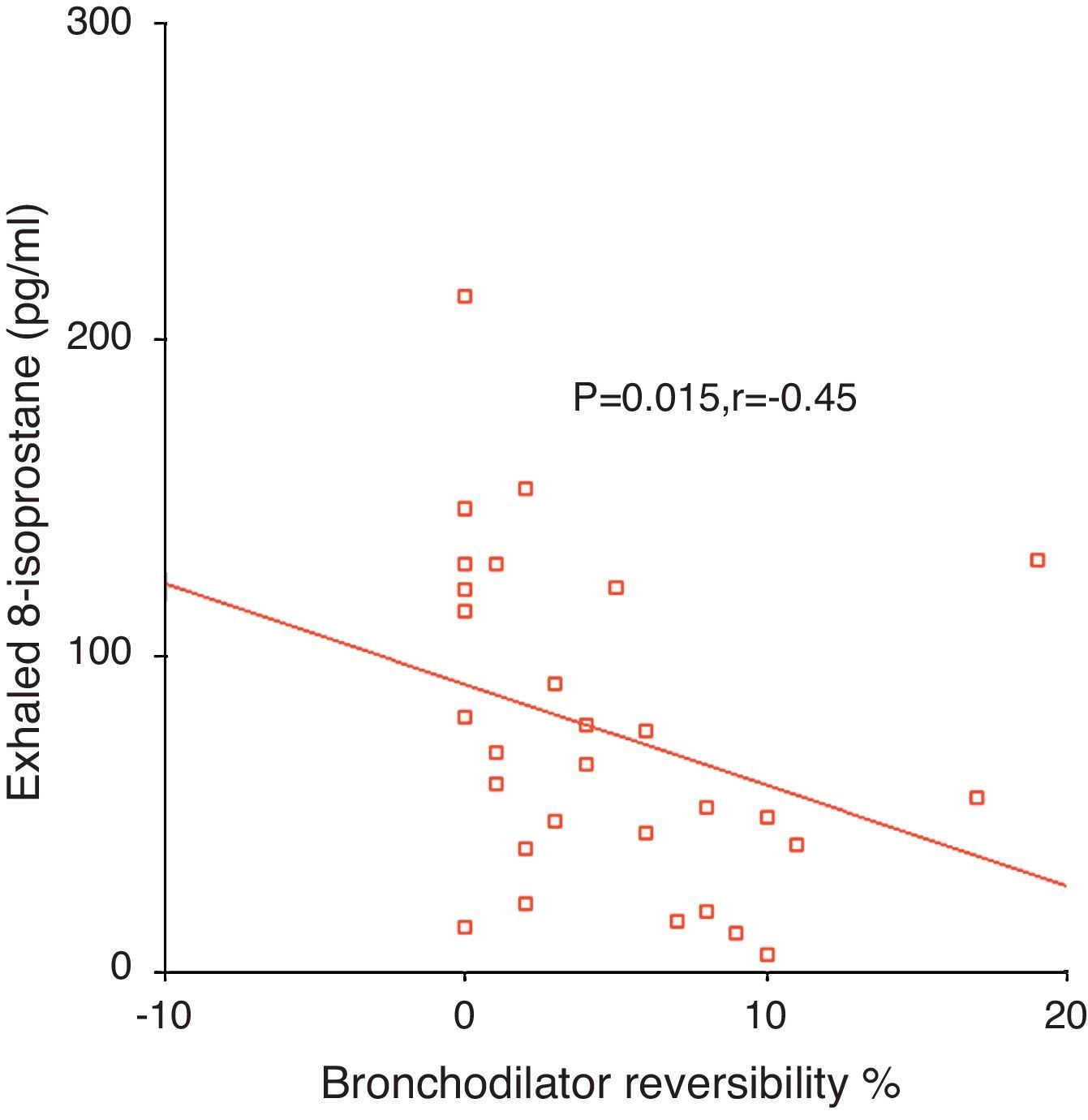
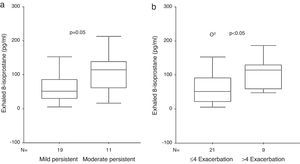
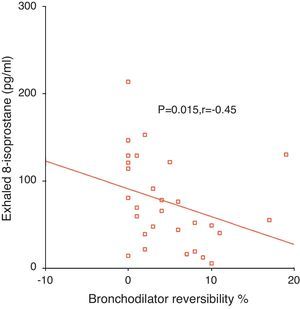
ResultsExhaled 8-isoprostane level in patients with moderate persistent asthma [114 (55–146)pg/ml] was higher than in the mild persistent group [52 (21–91)pg/ml] (p=0.05, Mann–Whitney U [MWU]). EBC 8-isoprostane in children with 1–4 asthma exacerbations/year [52 (16–80)pg/ml] was significantly lower than in children with >4 asthma exacerbations/year [114 (57–129)pg/ml] (p<0.05, MWU). No significant relation was determined between exhaled 8-isoprostane and Cys-LTs levels and c-ACT score and asthma control level. Exhaled 8-isoprostane correlated negatively with bronchodilator response (p=0.015, r=−0.45).
ConclusionsExhaled 8-isoprostane, as an oxidative stress specifier, was found to be increased in relation with asthma exacerbation frequency and oxidative stress increases with the severity of asthma. In contrast to asthma severity level, c-ACT score and asthma control level may not reflect airway inflammation.
Inflammation and oxidative stress are essential parts of the asthma pathophysiology. Cysteinyl leukotrienes (Cys-LTs) are potent constrictors and pro-inflammatory mediators that have been demonstrated to have a role in the asthma pathophysiology.1–6 Higher levels of Cys-LTs have been found in bronchoalveolar lavage, induced sputum, and in exhaled breath condensate (EBC) of asthmatics, especially in patients with unstable asthma.1–6
It was also shown that as a result of oxidative stress, 8-isoprostane, a good marker of oxidative stress due to its stability, specificity for lipid peroxidation, and in vivo production, increases in asthma in association with its severity.7–9
A variety of methods are used to measure intense inflammation in the airways of asthmatics.
EBC is a completely non-invasive method for the collection of airway secretions.10,11 It was demonstrated that exhaled Cys-LTs and 8-isoprostane measurements were increased in asthma.3–6,12
Recently, it has been shown that the asthma control test (ACT) is a good tool for use in the evaluation of asthmatic patients.13 ACT was found to be a clinically validated measure of asthma control which is simple to administer, and is useful for clinicians assessing asthma control in adult patients.13 Moreover, the childhood (c)-ACT score was developed to evaluate asthma control in children 4–11 years of age with asthma.14,15 Whether the c-ACT score and asthma control level correlate with airway inflammation in asthma is not well known. While there are studies investigating the relation between ACT score, asthma control level, asthma severity, and exhaled nitric oxide (eNO) in adults with asthma, and while it has been demonstrated that exhaled 8-isoprostane levels were significantly higher in severe compared to mild and moderate asthmatics adults, there has been no study assessing the correlation between c-ACT score, asthma control level, asthma severity, and exhaled Cys-LTs and 8-isoprostane levels at the same time in asthmatic children.12,16,17 Additionally, it is not well known among c-ACT score, asthma control level and asthma severity which correlates better with airway inflammation.
The aim of the present study was to establish the relation between exhaled Cys-LTs and 8-isoprostane levels and c-ACT score, asthma control level and asthma severity in children with asthma.
Materials and methodsSubjectsThe subjects were children aged 6–18 years who had asthma according to the criteria recommended by the Global Initiative for Asthma (GINA) updated in 2002.18 Twenty-eight children with mild-to-moderate persistent asthma had been on maintenance therapy with low-to-medium constant doses of inhaled corticosteroids (budesonide or fluticasone) for at least two months. None of the patients was being treated with leukotriene receptor antagonists.
Study designThirty children (24 males, 6 females) who applied to Gaziantep University Pediatric Allergy and Asthma Unit with asthma between January 2010 and December 2010 were included in our study.
All patients had been in a stable condition and free from acute exacerbations and respiratory tract infections in the previous two months when the evaluations were performed. All subjects were studied in the morning. Study participants sequentially undertook EBC collection, spirometry with a dry spirometer, assessment of total eosinophil and immunoglobulin (Ig)E levels, and the evaluation of sensitivity to commonly encountered aeroallergens (e.g. house dust mites, trees, weeds, grasses, cat, Alternaria, Cladosporium, egg white, cow's milk) by skin prick tests. The asthmatic children were evaluated with c-ACT score, and asthma severity and asthma control level were assessed according to GINA by paediatric allergists on the same day.18 All evaluations were performed once during the study. The child's family was asked to complete a questionnaire regarding c-ACT, the nature and duration of asthma symptoms, frequency of asthma exacerbation in the last year, and medication usage. An asthma exacerbation was defined as “episodes of progressive increase in shortness of breath, cough, wheezing, or chest tightness, or some combination of these symptoms, accompanied by decreases in expiratory airflow that can be quantified by measurement of lung function, unresponsive to the patient's routine asthma medication and additional β2 agonist therapy”.
The study was performed as a prospective trial. The personnel performing the enzyme linked immunosorbent assay (ELISA) tests were unaware of the clinical status of the case and protocol of the study.
The outcomes of the study were to establish the relation between exhaled Cys-LTs and 8-isoprostane levels and asthma severity, asthma control level and c-ACT score in children with asthma.
Study measurements and proceduresSpirometrySpirometric measurements were performed after collecting EBC by using a dry spirometer (Sensor Medics, Vmax22, CA, USA). The best of three forced expiratory volume in first second (FEV1) values was taken. A FEV1% of predicted reference values was calculated for each child. The bronchodilation response was assessed 15min after administration of 400μg salbutamol through a spacer (Volumatic®). The bronchodilation response was expressed as the relative increase of FEV1 compared to the predicted value of the FEV1.
Collection of EBCEBC was collected (ECoScreen, Jaeger, Höchberg, Germany) according to the American Thoracic Society/European Respiratory Society (ATS/ERS) Task Force recommendations.19 All subjects breathed in a relaxed manner (tidal breathing) for 15min not wearing a nose-clip. Approximately 1ml of breath condensate was collected in sterile Eppendorf tube which was then immediately stored at −80°C for later analysis.
Measurements of EBCExhaled 8-isoprostane concentrations were quantified using a specific enzyme immunoassay kit (Cayman Chemicals, Ann Arbor, MI, USA). The detection limit was 2.7pg/ml. Cys-LTs concentrations were determined using a specific immunoassay kit (Cayman Chemicals, Ann Arbor, MI, USA) as previously described. The detection limit was 13pg/ml.
EthicsAll study procedures were done in accordance with a protocol previously approved by the Ethics Committee of Gaziantep University. All parents provided written informed consent for the study procedures and the children gave their assent.
Statistical analysesA statistical software package was used for all data analysis and comparisons (SPSS v 11.5). The data were tested for assumptions of normality, and differences between the groups were compared with Mann–Whitney U (MWU) test and Kruskal–Wallis accompanied by Dunn's multiple comparison. Spearman's rank correlation coefficient was applied to investigate the correlation between different parameters. Multiple linear regressions were applied whenever needed to adjust for age and gender as potential confounders. A p value of less than 0.05 was considered significant.
Sample size was estimated using a power calculation based on 50% difference between groups in Cys-LTs and 8-isoprostane measurements. It was estimated that at least 12 patients would be required to detect a significant difference between groups at 80% power level and an alpha error of 5%.
ResultsThirty consecutive asthmatic children were tested. Characteristics of the asthmatic children are shown in Table 1. According to GINA classification, six patients (20%) were completely controlled, 15 patients (50%) were partly controlled, and nine patients (30%) were uncontrolled.
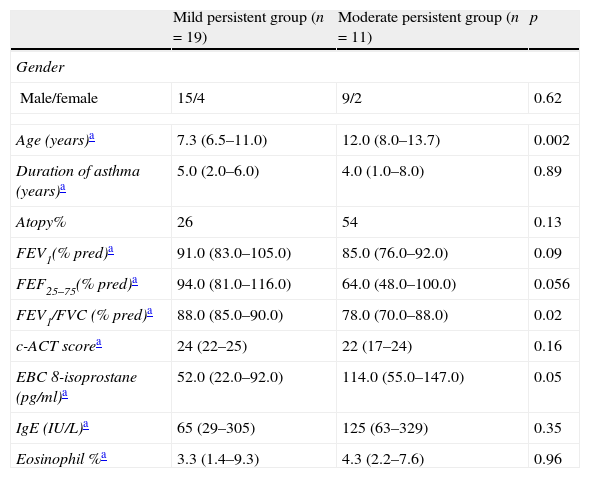
Main characteristics of the study population.
| Mild persistent group (n=19) | Moderate persistent group (n=11) | p | |
| Gender | |||
| Male/female | 15/4 | 9/2 | 0.62 |
| Age (years)a | 7.3 (6.5–11.0) | 12.0 (8.0–13.7) | 0.002 |
| Duration of asthma (years)a | 5.0 (2.0–6.0) | 4.0 (1.0–8.0) | 0.89 |
| Atopy% | 26 | 54 | 0.13 |
| FEV1(% pred)a | 91.0 (83.0–105.0) | 85.0 (76.0–92.0) | 0.09 |
| FEF25–75(% pred)a | 94.0 (81.0–116.0) | 64.0 (48.0–100.0) | 0.056 |
| FEV1/FVC (% pred)a | 88.0 (85.0–90.0) | 78.0 (70.0–88.0) | 0.02 |
| c-ACT scorea | 24 (22–25) | 22 (17–24) | 0.16 |
| EBC 8-isoprostane (pg/ml)a | 52.0 (22.0–92.0) | 114.0 (55.0–147.0) | 0.05 |
| IgE (IU/L)a | 65 (29–305) | 125 (63–329) | 0.35 |
| Eosinophil %a | 3.3 (1.4–9.3) | 4.3 (2.2–7.6) | 0.96 |
Exhaled 8-isoprostane levels were detected in 100% of the patients. Exhaled 8-isoprostane level in patients with moderate persistent asthma [114 (55–146)pg/ml] was higher than in the mild persistent group [52 (21–91)pg/ml] (p=0.05, MWU) (Fig. 1). EBC 8-isoprostane measurement in children with 1–4 asthma exacerbations/year [52 (16–80) pg/ml] was significantly lower than in children with >4 asthma exacerbations/year [114 (57–129)pg/ml] (p<0.05, MWU) (Fig. 1). Characteristics of the asthmatic children according to the frequency of asthma exacerbations are shown in Table 2.
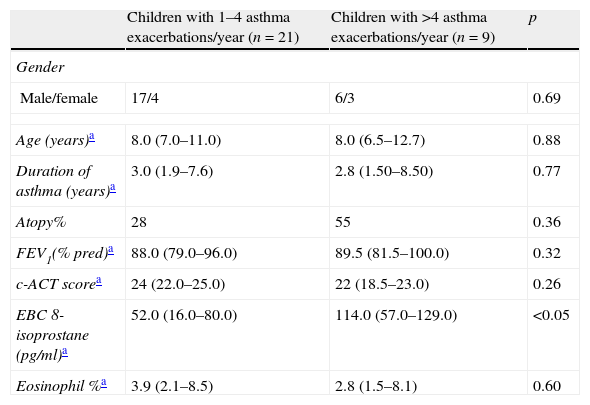
Main characteristics of the study population according to the frequency of asthma exacerbations/year.
| Children with 1–4 asthma exacerbations/year (n=21) | Children with >4 asthma exacerbations/year (n=9) | p | |
| Gender | |||
| Male/female | 17/4 | 6/3 | 0.69 |
| Age (years)a | 8.0 (7.0–11.0) | 8.0 (6.5–12.7) | 0.88 |
| Duration of asthma (years)a | 3.0 (1.9–7.6) | 2.8 (1.50–8.50) | 0.77 |
| Atopy% | 28 | 55 | 0.36 |
| FEV1(% pred)a | 88.0 (79.0–96.0) | 89.5 (81.5–100.0) | 0.32 |
| c-ACT scorea | 24 (22.0–25.0) | 22 (18.5–23.0) | 0.26 |
| EBC 8-isoprostane (pg/ml)a | 52.0 (16.0–80.0) | 114.0 (57.0–129.0) | <0.05 |
| Eosinophil %a | 3.9 (2.1–8.5) | 2.8 (1.5–8.1) | 0.60 |
Exhaled Cys-LTs levels were detected in 73% of the patients. Although there was a tendency for exhaled Cys-LTs levels to increase in children with moderate persistent asthma compared to the mild persistent group, no significant difference was found between the groups (data not shown).
No significant relation was detected between exhaled 8-isoprostane and Cys-LTs and c-ACT score, FEV1, or asthma control level.
Relationship between exhaled 8-isoprostane and bronchodilator responseExhaled 8-isoprostane levels correlated negatively with bronchodilator response (p=0.015, r=−0.45, Fig. 2).
Factors affecting exhaled 8-isoprostane levelsUnivariate analysis identified asthma severity level (odds ratio (confidence interval)) (2.27 (4.3–83.1)) and percentage changes in FEV1 after β2 agonist inhalation (−2.1 (−6.7)–(−0.78)) as significantly associated with exhaled 8-isoprostane levels (p=0.03 and 0.045, respectively). Results from multivariate regression analysis adjusted for age and asthma severity level as covariates showed that percentage changes in FEV1 after β2 agonist inhalation was significantly associated with exhaled 8-isoprostane measurements (−2.74 (−7.3)–(−1.4); p=0.01).
Factors affecting asthma severityUnivariate analysis identified age (1.64 (1.13–2.40)), exhaled 8-isoprostane levels (1.02 (1–1.03)) and FEV1/forced vital capacity (FVC) (0.86 (0.76–0.98)) as significantly associated with asthma severity levels (p=0.008, 0.045, 0.02, respectively). Results from multivariate regression analysis adjusted for age and exhaled 8-isoprostane level as covariates showed that FEV1/FVC was significantly associated with asthma severity (0.81 (0.67–0.98); p=0.03).
DiscussionAsthma is a complex disease of inflamed airways with varying severity and control level. The severity and control level of asthma are decided based upon clinical symptoms. Guidelines recommend us to treat patients with asthma according to the asthma severity at the beginning of treatment and to continue therapy based on the control of asthma.18 Even if asthma treatment is planned according to the clinical features and pulmonary function tests, asthma symptoms may not adequately show airway inflammation and pulmonary function tests may indicate inflammation to a limited degree.20–22 Although some medications decrease asthma symptoms and increase the level of asthma control, inflammation may not be reduced. These results may indicate that by measuring airway inflammation, we can decide to apply other treatment regimens like antioxidant treatment in addition to the standard therapy.
Exhaled breath condensate (EBC) is one of the most non-invasive methods for the collection of airway secretion droplets and for measuring inflammation directly from airway secretions from airway epithelia and/or inflammatory cells. Compared to bronchoalveolar lavage and induced sputum, it is harmless in asthmatic children.
Eosinophils, neutrophils, macrophages, and mast cells all produce reactive oxygen radicals causing an increase in oxidative stress as a feature of airway inflammation in asthma.8 8-Isoprostane is a stable product formed by oxidative metabolism of arachidonic acid and seems to be a reliable sign of oxidative stress.9 It has been shown that exhaled 8-isoprostane is elevated in asthmatic children, and that this does not seem to be normalised by inhaled steroid therapy.6,23,24 Exhaled 8-isoprostane, as an oxidative stress specifier, was found to be increased in relation with asthma exacerbation frequency in our study. Asthma exacerbations remain an important health problem, which in itself is a risk factor for severe fixed airway obstruction. When asthmatic children have increased asthma exacerbation, they may face more oxidative stress. As a consequence of asthma exacerbation, exhaled 8-isoprostane levels increase. All these findings indicate that physicians should try to prevent asthma exacerbations to the greatest extent possible with regular prophylaxis to reduce oxidative stress and prevent irreversible changes in the airways of asthmatics. Furthermore, we showed that when exhaled 8-isoprostane level increases, bronchoreversibility decreases. Oxidative stress may prevent the dilatation ability of bronchial smooth muscle in response to inhaled salbutamol. In addition, our study showed that oxidative stress increases with the severity of asthma. Comparison of 8-isoprostane levels revealed that exhaled 8-isoprostane levels were higher in children with moderate persistent asthma compared to the mild persistent asthma group. Similar to our results, Robroeks et al. showed that exhaled 8-isoprostane was related to both asthma severity and control in children with asthma.25 They showed that exhaled 8-isoprostane, interferon (IFN)-γ, interleukin (IL)-4, and fractional (F)eNO are discriminative markers between good asthma and poor asthma control, and asthma severity was best indicated by 8-isoprostane, nitrate, nitrite, and FeNO. However, we could determine no significant relation between asthma control level and the exhaled mediators. Indeed our study was underpowered with respect to the number of patients in each asthma control level while investigating the relation between asthma control level and exhaled mediators. Our results must be tested by studies with a higher number of patients with a wider range of asthma control level.
Furthermore, the c-ACT is a simple, patient-based tool providing continuous assessment of asthma control; however, its relation with inflammation is yet to be demonstrated. Consequently, we could not show any significant relation between c-ACT score and exhaled 8-isoprostane or Cys-LTs. Accordingly, Piacentini et al. showed that the assessment of asthma control by c-ACT is not related to the level of FeNO in asthmatic children under regular follow-up.16 Moreover, it has been shown in a recently published study that ACT score significantly correlated with symptom score, rescue medication use, and peak expiratory flow (PEF) variability, but not with FEV1 and markers of airway inflammation, regardless of inhaled corticosteroid treatment.26 On the other hand, a previous study showed that only 10% of asthmatic patients presented with both an ACT <19 and an FEV1 <80% of predicted.27 These data reinforced the clear GINA advice that questionnaires intended for the level of asthma control need to be considered along with the results of lung function measurements.18 Supporting this recommendation, our study showed that while asthma severity level was correlated with exhaled 8-isoprostane levels, the c-ACT score was not. One explanation for this finding is the use of pulmonary function measurements in the evaluation of asthma severity, but not in the determination of the c-ACT score.
With regard to our finding of a discrepancy between the c-ACT score and exhaled 8-isoprostane, the following may be mentioned as probable contributing factors: first, poor perception of symptoms is a major problem in asthmatics, and the c-ACT score consists of symptoms irrespective of pulmonary function.28 Second, while long-term asthma treatment with inhaled corticosteroids decreases asthma symptoms, it may not decrease exhaled 8-isoprostane levels as shown before.24 Third, while the c-ACT score shows the severity of symptoms in the latest four-week period, exhaled 8-isoprostane levels may be related with the baseline characteristic of the disease. Fourth, we evaluated asthmatic children who were asymptomatic for two months, which prevented the assessment of asthmatics with low c-ACT score.
As suggested by Piacentini et al.,16 we may interpret our findings as those who are c-ACT controlled/exhaled 8-isoprostane uncontrolled are symptomatically controlled, but have evidence of persistent inflammation and could be at risk for future asthma problems, such as asthma exacerbation.
To our knowledge, no previous study has investigated the correlation between c-ACT score and exhaled 8-isoprostane and Cys-LTs concentrations in asthmatic children. In the light of our results, the relationship between the c-ACT score and airway inflammation should be investigated in multicentre studies.
Because most of our patients were using inhaled corticosteroids, we could not show any relation between exhaled Cys-LTs and asthma severity and asthma control level. In this regard, we recommend that exhaled Cys-LTs should be measured by more sensitive immunoassay kits with lower detection limits. In addition there are missing cases in the measurements of exhaled Cys-LTs, which reduce the overall number of subjects and increase the risk of a type II error with the ensuing impact on the inferences.
The current limitation of the EBC analysis is the deficit of validation of the immunoassays for exhaled markers with more specific analytic techniques. Fortunately, it was confirmed that exhaled leukotrienes were increased in asthmatics using gas chromatography–mass spectrometry, which is the reference analytical technique.4 Moreover, the specificity of radioimmunoassays (RIAs) for 8-isoprostane was demonstrated by reverse phase-high performance liquid chromatography (RP-HPLC).29,30 Furthermore, day-to-day repeatability of exhaled eicosanoids was demonstrated in previous studies.24,31 In the present study, we tried to decrease the variability in exhaled mediators by having the same personnel collect the EBC, at a standard volume and duration, and by using the same analytical technique and evaluating all patients in the morning.
ConclusionsIn conclusion, supporting previous studies, our study reinforced that exhaled 8-isoprostane is a successful and reliable mediator in determining asthma severity even in asthmatic children on maintenance treatment for asthma. Furthermore, in contrast to asthma severity, c-ACT score and asthma control level may not reflect airway inflammation in asthma. These results should be supported by studies with a higher number of asthmatics with a wider range of asthma control level and c-ACT score.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
Confidentiality of dataThe authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.
Declaration of interestThis study was funded by a grant from Gaziantep University (no. TF.09.18).
Conflict of interestThe authors have no conflict of interest to declare.
The authors thank Sevil Kanat and Sevda Korkut for their technical assistance.