Pollen food syndrome is one of the main causes of food allergies in adults. However, the intrinsic immunological mechanisms remain unclear.
MethodsForty pollinosis sufferers [23 with a food allergy (PSFA) and 17 without a food allergy (PS)] and 17 non-atopic healthy controls were included in this study. The PSFA group was subdivided into an oral allergy syndrome group, a systemic reaction group, and an anaphylactic reaction group according to their symptoms after eating the suspected foods. Serum IL-10 and TGF-β levels of all participants were determined by ELISA. Clinical characteristics of the patients were also evaluated.
ResultsThere were no significant differences in age, sex, pollen-associated symptoms, duration of respiratory disease, and positive parental history of atopy between the PSFA and PS groups. Compared to healthy controls, serum IL-10 levels of both the PSFA group and PS group were significantly lower (p≤0.01), but TGF β levels were significantly higher in the PSFA group (35.3±5.6ng/ml vs. 31.2±6.6ng/ml, respectively; p=0.037). Within the PSFA group, IL-10 levels in the anaphylactic reaction subgroup were significantly lower compared to oral allergy syndrome subgroup (1.87±0.47pg/ml vs. 1.40±0.30pg/ml, respectively; p=0.027). More severe food allergy symptoms were associated with lower serum IL-10 levels. In contrast, the highest serum levels of TGF-β were found in patients from the anaphylactic reaction subgroup.
ConclusionsWith the exception of a defect in regulatory cells represented by the reduction of IL-10, other potential immunological mechanisms (e.g., Th17 or IL-23 together with TGF-β) may be involved in the development of pollen food syndrome.
The prevalence of food allergy is increasing throughout the world. Food-induced allergic reactions range from mild discomfort restricted to the oral cavity, to systemic and severe symptoms, such as anaphylaxis. Foods are the most common triggers of severe anaphylaxis.1
There are typically two types of food allergies: one is primary sensitisation, which initiates in the gastrointestinal tract, and the other is secondary sensitisation, which is a result of cross-reactivity to inhalant allergens. Primary sensitisation is most frequently seen in children and is usually the first manifestation of the atopic march (ranging from food allergy-related eczema/urticaria to allergic rhinitis and then asthma). Pollen is the most common cross-reactive inhalant allergen involved in plant food allergies.
Pollen-related food allergy, also called ‘pollen food syndrome’, is very common, and even represents the predominant type of food allergy in some countries.2,3 It was first described by Ortolani et al. in 1988 who noticed that the symptoms were limited to oral allergy reactions.4 Thereafter, it became known that oral discomfort after consumption of fruits or vegetables may be just a premonition to a variety of generalised symptoms, including life-threatening anaphylaxis.
The extensive presence of pan-allergens (e.g., PR-10s, nsLTPs, profilins, and others) from taxonomically-related or unrelated species sharing highly preserved structures and functions accounts for the high incidence of cross-reactivity between pollens and plant foods.5 An accurate prevalence of pollen food syndrome is difficult to estimate, because many people who suffer from mild discomfort after eating the implicated food may simply avoid it instead of reporting the symptoms to a physician. According to previous reports, approximately 40–70% of pollinosis sufferers develop a relevant plant food allergy.6–9 These findings suggest that a substantial number of patients who are allergic to pollen do not develop a food allergy later in life. There may also be some genetic or immunological differences between patients with hay fever with and without pollen food syndrome. Cuesta-Herranz et al. previously compared the clinical and laboratory characteristics of pollen-allergic patients with and without a food allergy. They found that pollen-allergic patients with an associated plant food allergy had a higher frequency of asthma, higher frequency of polysensitisation, and a different pattern of specific IgEs to several kinds of pollen allergens. In addition, patients sensitised to pollen from Platanus, Platago, Artemisia, Betula, Parietaria, and Salsola were predisposed to the development of a plant food allergy.6 Peach was found to have the highest frequency of allergy among the relevant plant foods in their study.
Recently, the close relationship between regulatory T cells and oral tolerance and food allergy has been well characterised.10–13 There are two types of regulatory T cells: natural regulatory T cells (nTreg), which are generated in the thymus expressing high level of Foxp3, and inducible T cells (iTreg), which are converted from naïve T cells in the periphery. The latter includes at least two subtypes: IL-10-secreting type 1 regulatory T cells (Tr1)14 and TGF-β-producing Th3 regulatory T cells.15 IL-10 and TGF-β are the main effector molecules of regulatory T cells and play an important role in the maintenance of immune homeostasis throughout the entire body.
This study aimed to determine serum levels of IL-10 and TGFβ in pollinosis patients with and without a food allergy and to investigate their potential relationship with the development of pollen food syndrome.
Materials and methodsSubjectsForty patients suffering from Artemisia pollinosis and/or birch pollinosis [23 with a food allergy (PSFA) and 17 without a food allergy (PS)], and 17 non-atopic healthy controls were included in this study. PSFA patients were subdivided into an oral allergy syndrome group (n=8), systemic reaction group (n=6), and anaphylactic reaction group (n=9) according to the severity of symptoms after consuming the suspected foods. Clinical characteristics of the patients, including age, sex, pollen-associated symptoms, food-related symptoms, duration of respiratory disease, and positive parental history of atopy, were also evaluated.
Anaphylactic reaction, namely anaphylaxis, was defined as rapid-onset, multisystem, severe, or even life-threatening reactions occurring upon allergen exposure based on World Allergy Organization (WAO) guidelines.16 Systemic reactions showing symptoms that were restricted to only one system, such as respiratory, gastrointestinal, or skin/mucosa, did not meet the diagnosis criteria of anaphylaxis.
This research protocol was approved by the Ethical Committee of Peking Union Medical College Hospital. Written informed consent was obtained from each participant.
Inclusion criteriaInclusion criteria included seasonal symptoms of allergic rhinitis and/or asthma; confirmed sensitisation to birch and/or mugwort pollen by a serum-specific IgE test and skin prick test; plant food allergy diagnosed on the basis of compelling history, including repetitive adverse reactions to certain plant foods and no occurrence of relevant discomfort after complete avoidance of the same food; and a positive serum-specific IgE test against the suspected food.
Exclusion criteriaAll patients included in this study were generally healthy. Patients with serious cardiac and vascular diseases, liver problems, renal dysfunction, mental disorders, tumours, autoimmune diseases, and infectious diseases as well as other types of atopic disease, such as eczema or chronic urticaria, were excluded. Patients who had ever received immunotherapy before recruitment onto the study were also excluded. No glucocorticoids or analogues were allowed one month prior to blood collection.
Sample preparationFour millilitres of peripheral venous blood were collected from each subject. Serum was isolated by centrifuging the samples at 3900rpm for 5min after allowing them to stand at room temperature for 30min. After centrifugation the samples were frozen at −80°C for further analysis.
Serum analysisSerum IL-10 and TGF-β levels of all participants were detected using a commercial ELISA kit (eBioscience, USA). The detection limitation was 0.39pg/ml for IL-10 and 63pg/ml for TGF-β. The sensitivity of the two kits were 0.05pg/ml and 15pg/ml, respectively. Total IgE and specific IgE levels were detected by ImmunoCAP system (Thermo Fisher, Uppsala, Sweden).
Statistical analysisThe statistical software SPSS 16.0 (SPSS, Inc., Chicago, IL, USA) was used to analyse the data. Frequencies of the qualitative variables were compared by Pearson's chi-squared test or Fisher's exact test. The comparison of quantitative variables was carried out using the Student's t-test in case of normally distributed data and the Mann–Whitney U-test in case of non-normally distributed data. A p value<0.05 was considered statistically significant.
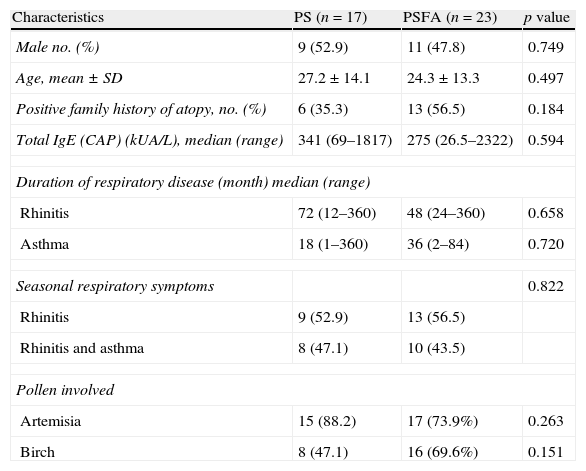
ResultsAll 40 patients with hay fever were from northern China. Birch and Artemisia pollen was the most common trigger of pollinosis in spring and late summer/early autumn, respectively. The clinical characteristics of the patients are shown in Table 1. No significant difference was found in sex, age, family history of atopy, serum total IgE levels, duration of respiratory disease, or pollen-related respiratory symptoms between the PS and PSFA groups. The average age of healthy controls was 27±15.1 years, which included 11 females and six males. There were no differences in the age and sex ratios between healthy controls (HC) and PS or PSFA patients.
Clinical characteristics of patients included in this study.
| Characteristics | PS (n=17) | PSFA (n=23) | p value |
| Male no. (%) | 9 (52.9) | 11 (47.8) | 0.749 |
| Age, mean±SD | 27.2±14.1 | 24.3±13.3 | 0.497 |
| Positive family history of atopy, no. (%) | 6 (35.3) | 13 (56.5) | 0.184 |
| Total IgE (CAP) (kUA/L), median (range) | 341 (69–1817) | 275 (26.5–2322) | 0.594 |
| Duration of respiratory disease (month) median (range) | |||
| Rhinitis | 72 (12–360) | 48 (24–360) | 0.658 |
| Asthma | 18 (1–360) | 36 (2–84) | 0.720 |
| Seasonal respiratory symptoms | 0.822 | ||
| Rhinitis | 9 (52.9) | 13 (56.5) | |
| Rhinitis and asthma | 8 (47.1) | 10 (43.5) | |
| Pollen involved | |||
| Artemisia | 15 (88.2) | 17 (73.9%) | 0.263 |
| Birch | 8 (47.1) | 16 (69.6%) | 0.151 |
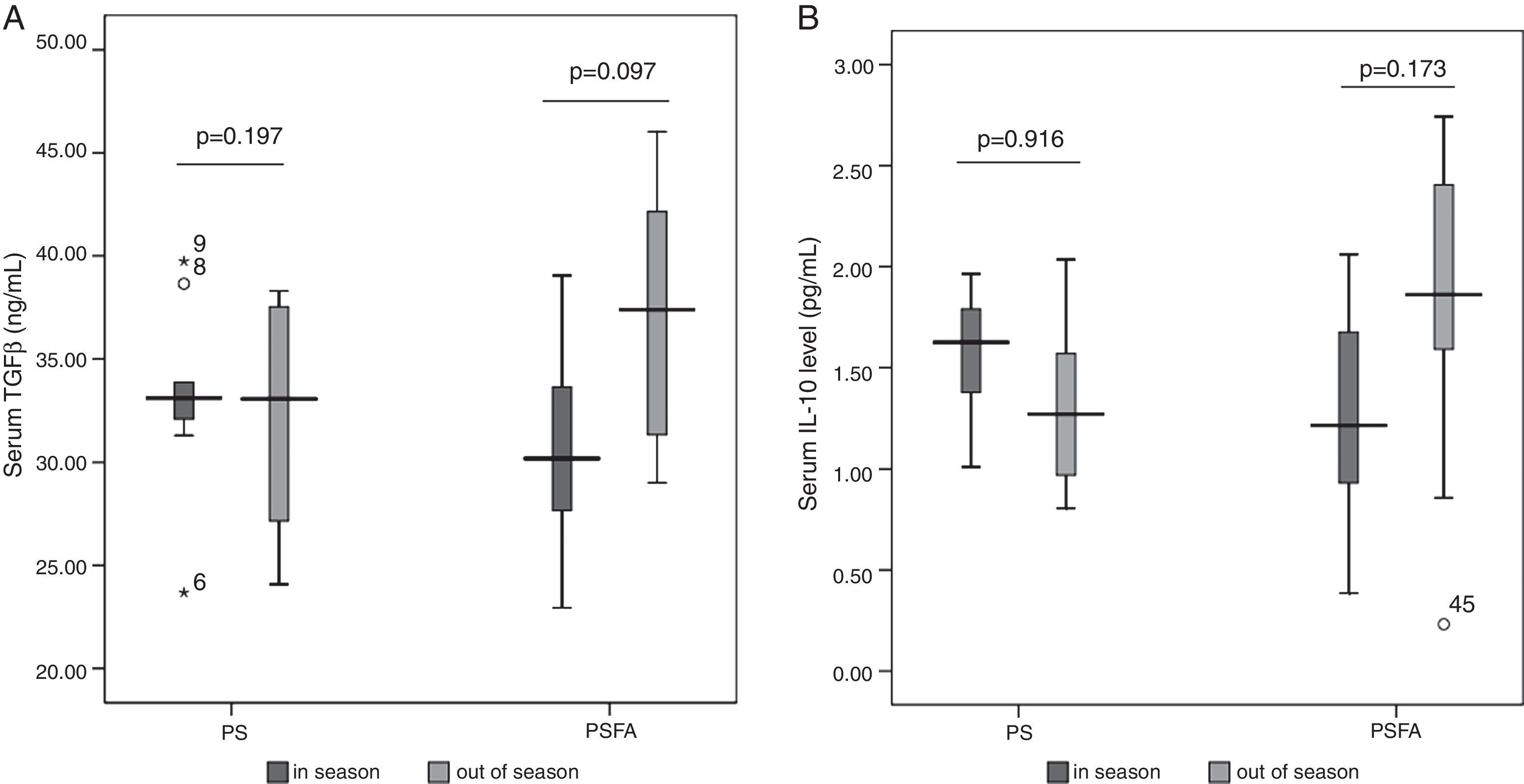
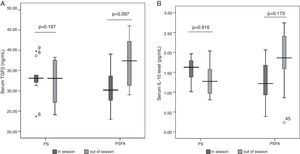
Among the total 40 pollinosis patients, seven were only allergic to birch, 16 were only allergic to Artemisia, and 17 were allergic to both. Among the 23 pollinosis patients with a food allergy, six were allergic to birch pollen, seven were allergic to Artemisia pollen, and 11 were allergic to both. The frequencies of Artemisia and birch pollen allergies in the PS group were equal to those in the PSFA group. Among the PS group, nine patients were recruited within the allergy seasons and eight were recruited in the non-allergy seasons. Among the PSFA group, 12 were recruited within the allergy seasons and 11 were recruited in the non-allergy seasons. There was no difference in the percentage of patients recruited within allergy or non-allergy seasons in both groups (Pearson's χ2, p=0.962).
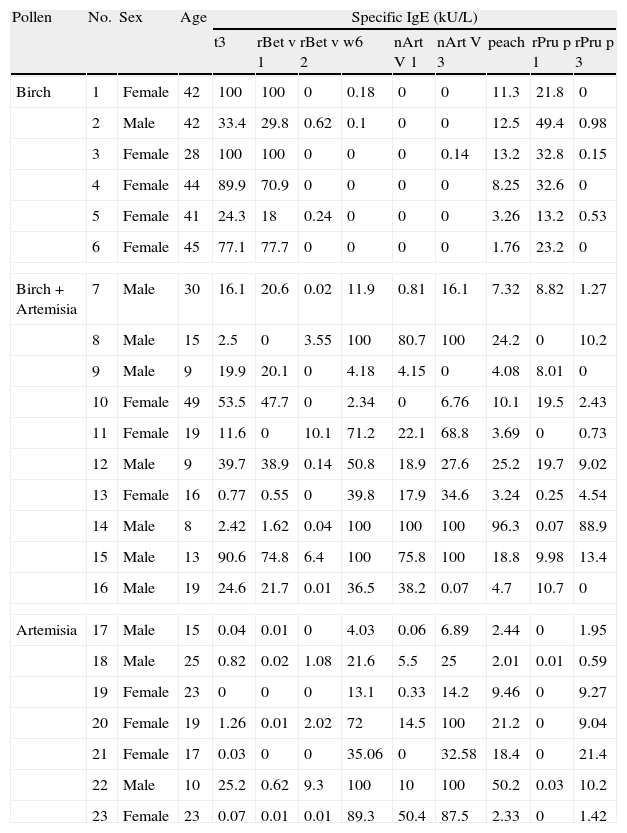
All patients in the PSFA group were allergic to peach (100%), while some of them were also allergic to other plant foods with less frequency, such as apple (69.6%); pear (30.4%); Chinese date (30.4%); apricot (26.1%); mango (21.7%); and hazelnut (21.7%). The sensitisation profiles of the 23 pollinosis sufferers with a plant food allergy are shown in Table 2. All six patients who were only allergic to birch pollen were sensitised to rBet v 1 and its homologue, rPru p 1. Among the seven patients only allergic to Artemisia pollen, all were sensitised to nArt v 3 and rPru p 3. Although a positive IgE response to Birch pollen was identified in three of the patients, none of them were sensitised to the relevant food allergen rPru p 1. The 10 patients allergic to both birch and Artemisia pollen showed a mixed sensitisation profile. Only four patients were simultaneously sensitised to rBet v 1 and nArt v 3 as well as their respective homologues rPru p 1 and rPru p 3. Two patients were sensitised to rBet v 2, nArt v 3, and rPru p 3. Two were only sensitised to rBet v 1 and rPru p 1, and the last two were sensitised to rBet v 1, nArt v 3and rPru p 3, but did not show positive response to rPru p 1, the homologue of rBet v 1.
Basic information and sensitisation profiles of pollinosis sufferers with a plant food allergy.
| Pollen | No. | Sex | Age | Specific IgE (kU/L) | ||||||||
| t3 | rBet v 1 | rBet v 2 | w6 | nArt V 1 | nArt V 3 | peach | rPru p 1 | rPru p 3 | ||||
| Birch | 1 | Female | 42 | 100 | 100 | 0 | 0.18 | 0 | 0 | 11.3 | 21.8 | 0 |
| 2 | Male | 42 | 33.4 | 29.8 | 0.62 | 0.1 | 0 | 0 | 12.5 | 49.4 | 0.98 | |
| 3 | Female | 28 | 100 | 100 | 0 | 0 | 0 | 0.14 | 13.2 | 32.8 | 0.15 | |
| 4 | Female | 44 | 89.9 | 70.9 | 0 | 0 | 0 | 0 | 8.25 | 32.6 | 0 | |
| 5 | Female | 41 | 24.3 | 18 | 0.24 | 0 | 0 | 0 | 3.26 | 13.2 | 0.53 | |
| 6 | Female | 45 | 77.1 | 77.7 | 0 | 0 | 0 | 0 | 1.76 | 23.2 | 0 | |
| Birch+Artemisia | 7 | Male | 30 | 16.1 | 20.6 | 0.02 | 11.9 | 0.81 | 16.1 | 7.32 | 8.82 | 1.27 |
| 8 | Male | 15 | 2.5 | 0 | 3.55 | 100 | 80.7 | 100 | 24.2 | 0 | 10.2 | |
| 9 | Male | 9 | 19.9 | 20.1 | 0 | 4.18 | 4.15 | 0 | 4.08 | 8.01 | 0 | |
| 10 | Female | 49 | 53.5 | 47.7 | 0 | 2.34 | 0 | 6.76 | 10.1 | 19.5 | 2.43 | |
| 11 | Female | 19 | 11.6 | 0 | 10.1 | 71.2 | 22.1 | 68.8 | 3.69 | 0 | 0.73 | |
| 12 | Male | 9 | 39.7 | 38.9 | 0.14 | 50.8 | 18.9 | 27.6 | 25.2 | 19.7 | 9.02 | |
| 13 | Female | 16 | 0.77 | 0.55 | 0 | 39.8 | 17.9 | 34.6 | 3.24 | 0.25 | 4.54 | |
| 14 | Male | 8 | 2.42 | 1.62 | 0.04 | 100 | 100 | 100 | 96.3 | 0.07 | 88.9 | |
| 15 | Male | 13 | 90.6 | 74.8 | 6.4 | 100 | 75.8 | 100 | 18.8 | 9.98 | 13.4 | |
| 16 | Male | 19 | 24.6 | 21.7 | 0.01 | 36.5 | 38.2 | 0.07 | 4.7 | 10.7 | 0 | |
| Artemisia | 17 | Male | 15 | 0.04 | 0.01 | 0 | 4.03 | 0.06 | 6.89 | 2.44 | 0 | 1.95 |
| 18 | Male | 25 | 0.82 | 0.02 | 1.08 | 21.6 | 5.5 | 25 | 2.01 | 0.01 | 0.59 | |
| 19 | Female | 23 | 0 | 0 | 0 | 13.1 | 0.33 | 14.2 | 9.46 | 0 | 9.27 | |
| 20 | Female | 19 | 1.26 | 0.01 | 2.02 | 72 | 14.5 | 100 | 21.2 | 0 | 9.04 | |
| 21 | Female | 17 | 0.03 | 0 | 0 | 35.06 | 0 | 32.58 | 18.4 | 0 | 21.4 | |
| 22 | Male | 10 | 25.2 | 0.62 | 9.3 | 100 | 10 | 100 | 50.2 | 0.03 | 10.2 | |
| 23 | Female | 23 | 0.07 | 0.01 | 0.01 | 89.3 | 50.4 | 87.5 | 2.33 | 0 | 1.42 | |
Using an IL-10 ELISA kit with high sensitivity, the serum IL-10 levels of only one patient in the PSFA group were lower than the detection limit for the assay. Because the OD value for this sample was very close to the detection limit, we also calculated the value using the formula deduced from the standard curve. The serum levels of TGF-β were detectable in all patients and healthy controls.
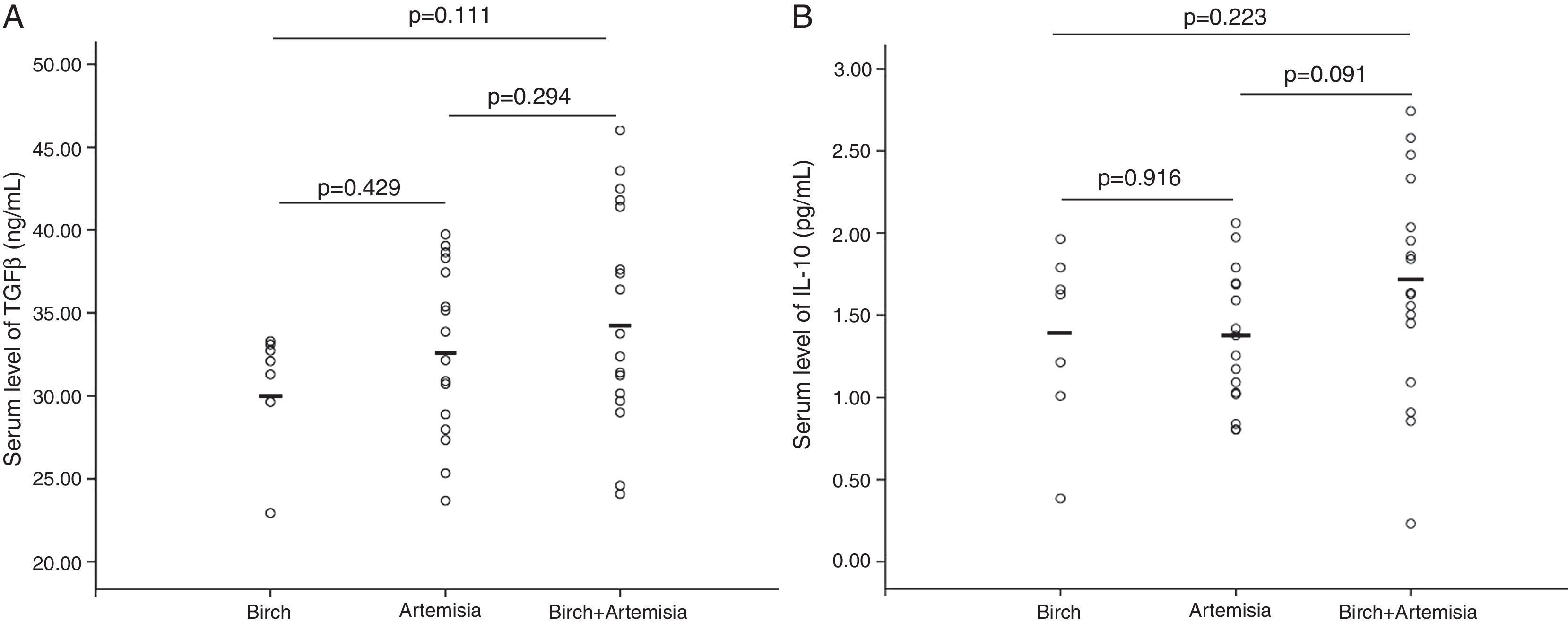
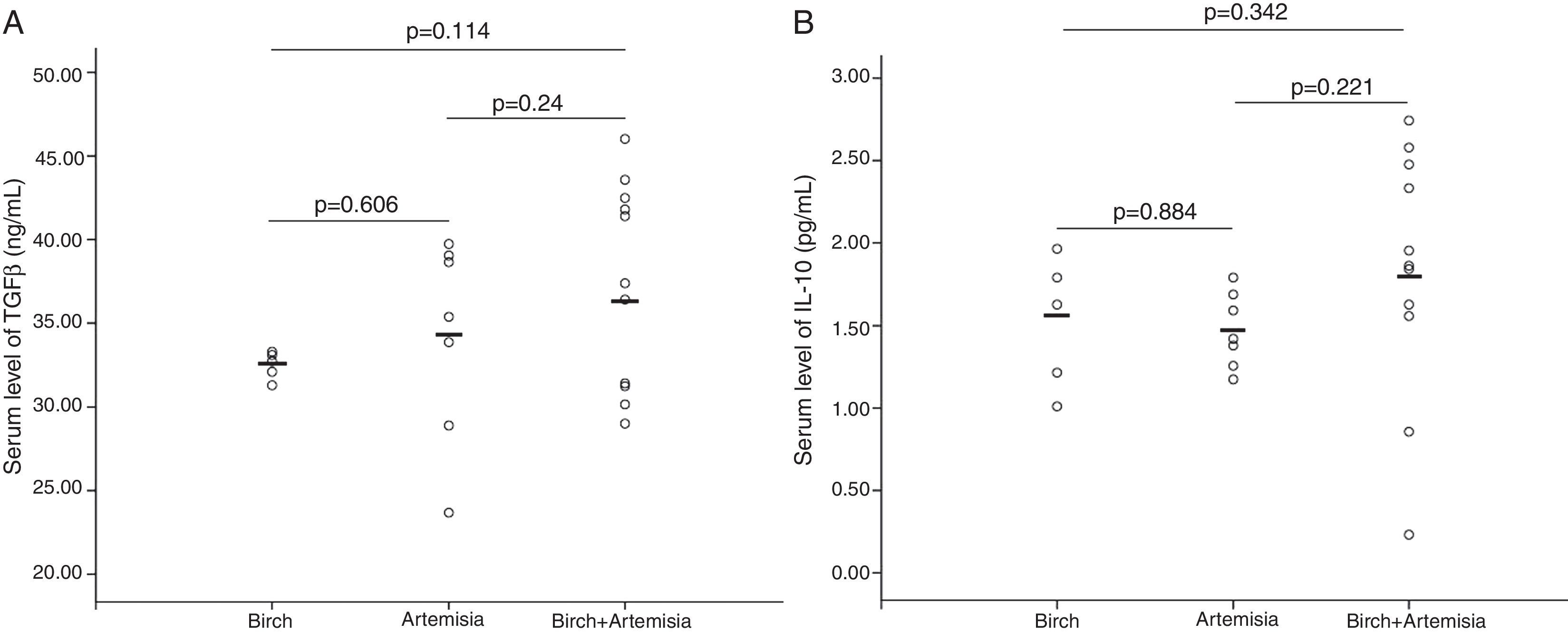
No differences in serum levels of IL-10 or TGF-β were found among patients allergic to different types of pollens (Figs. 1 and 2) or patients recruited at different times (Fig. 3). In both the PS and PSFA groups, no significant differences were found in serum levels of IL-10 and TGF-β between patients with and without asthmatic symptoms (p>0.05).
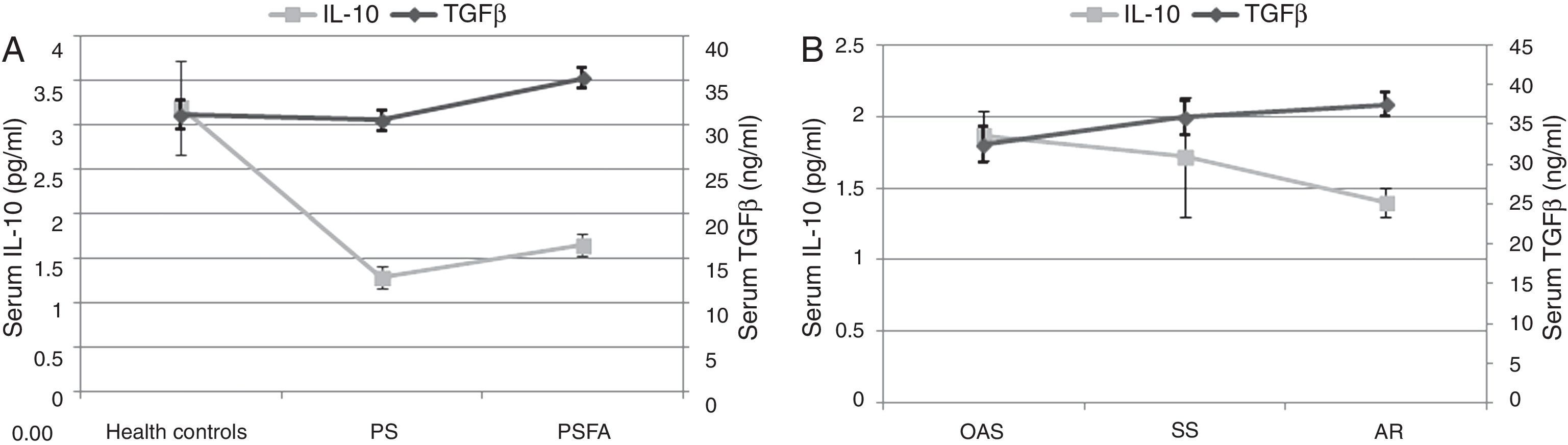
Compared to healthy controls, the serum IL-10 levels of both the PSFA and PS groups were significantly lower (p≤0.01), but TGF-β was significantly higher only in the PSFA group (35.3±5.6ng/ml vs. 31.2±6.6ng/ml, respectively; p=0.037). No significant difference was found in IL-10 levels between the PSFA and PS groups (1.66±0.60pg/ml vs. 1.29±0.50pg/ml, respectively; p=0.07), but serum TGF-β levels in the PSFA group were markedly higher than those in the PS group (35.3±5.6ng/ml vs. 30.6±4.8ng/ml, respectively; p=0.008). Within the PSFA group, IL-10 levels in the anaphylactic reaction subgroup were significantly lower compared to the oral allergy syndrome subgroup (1.87±0.47pg/ml vs. 1.40±0.30pg/ml, respectively; p=0.027). We found that more severe food allergy symptoms corresponded to lower serum IL-10 levels. However, in contrast, the highest serum TGF-β levels were observed in the anaphylactic reaction subgroup (Fig. 4).
DiscussionPollen food allergy syndrome is a main cause of adult food allergies in many countries, and food-associated symptoms are often serious and perennial.17 Therefore, this condition substantially affects the quality of life of these sufferers. This study investigated the intrinsic mechanisms of pollen food syndrome, which may help identify a useful preventive and therapeutic approach for this burdensome disorder. Peach is one of the most common allergenic fruits involved in pollen food syndrome, and its component allergens, Pru p 1, Pru p 3, and Pru p 4, are all cross-reactive and belong to the PR-10, nsLTP, and Profilin families, respectively. Thus, peach was chosen to be a model food in this study to analyse the potential cross-reactivity between pollens and foods. As shown in Table 2, birch pollinosis patients with a peach allergy were usually sensitised to rBet v 1 and its homologous allergen rPru p 1, and patients allergic to mugwort pollen and peach were simultaneously sensitised to nArt v 3 and its homologue rPru p 3. Moreover, patients allergic to both birch and mugwort pollen displayed mixed sensitisation patterns of peach.
Our data showed that no differences in age, sex, family history of atopy, serum total IgE levels, ratio of patients with asthmatic symptoms, duration of pollen-related respiratory disease, or type of allergenic pollen were found between the PS and PSFA groups in this study. However, serum levels of IL-10 and TGF-β were significantly different between the groups. Based on the severity of the food allergy symptoms, the levels of the two cytokines varied inversely; that is, patients with more severe symptoms had higher TGF-β levels and lower IL-10 levels. Serum IL-10 and TGF-β levels are the most important effecter molecules of regulatory T cells, which play a crucial role in maintaining the immunological balance throughout the body.
Although pollen food syndrome is different from a type 1 food allergy, which is primarily sensitised in the gut, the more important triggering phase of both types of food allergy occurs in the gastrointestinal tract. The immune environment of the gut is also crucial for the development and subsequent clinical manifestations of pollen-related food-induced disorders.
Normally, the gastrointestinal tract remains in a state of immunological homeostasis. Immunocytes and immune factors present in the gastrointestinal tract react correctly to the pathogens and tolerate food antigens and commensal bacteria well. All types of regulatory T cells are involved in the maintenance of immune balance of the gut. Foxp3+ nTregs generated in the thymus can migrate to other tissues, including the gastrointestinal tract, to exert suppressive function with the help of a spectrum of homing receptors, such as gut-homing integrin and α4β7. The iTregs generated in the gut, such as Tr1 and Th3T cells, could also enter the circulation and reach other organs to help maintain immune tolerance in those organs. This phenomenon is called ‘oral tolerance’, which has been shown to have the potential to prevent and treat autoimmune disease.13
TGF-β, a powerful immunosuppressive cytokine essential for the establishment of oral tolerance, is abundantly secreted by gut immunocytes upon oral antigen exposure. It can induce the expression of Foxp3, which is the key gene controlling the development and functioning of nTregs,18 and can convert the conventional CD4+CD25− T cells to CD4+CD25+ regulatory T cells.19,20 Moreover, TGF-β can inhibit the expression of the lineage-specific transcription factors T-bet and GATA-3 thereby blocking the differentiation of Th1 and Th2 cells, respectively.21 The contribution of TGF-β in maintaining the homeostasis of the gut is also displayed in its capacity to promote IgA class switching.22
In addition, TGF-β is a profibrotic cytokine that is crucial for airway wound repair. However, excessive activation of TGF-β can promote airway fibrosis and ultimately leads to airway remodelling.23 Protein and mRNA gene expression of TGF-β are generally increased in asthmatic patients. In our study, the duration of respiratory diseases and number of patients with asthma in the PS group was comparable to that in the PSFA group. Therefore, the impact of these factors on the findings of this study was excluded from the analysis. No difference was found in TGF-β levels between patients with only seasonal rhinitis or patients with both rhinitis and asthma, which could be due to the fact that all patients appeared healthy and only presented pollen-related symptoms during limited periods of the year. The actual duration of symptoms and inflammatory status of the airway is quite shorter than that of the patients with persistent rhinitis and or asthma.
IL-10 is another multifunctional modulatory cytokine.24 IL-10 can downregulate the expression of the IgE receptor and its signal molecules, including Syk, Fyn, and Akt, which alleviates the inflammatory reaction caused by IgE. It can also induce the differentiation of naïve T cells to Tr1 cells and maintain the expression of Foxp3 in nTregs. In addition, IL-10 is capable of promoting the generation of IgG4, which is always thought to be a blocking antibody that represents a state of tolerance.
Previous studies have suggested that gene polymorphisms and peripheral expression of IL-10 are associated with food allergies.25,26 We previously found that a single nucleotide polymorphism in the IL-10 gene was associated with the occurrence of anaphylaxis (data not published). Moreover, in a mouse model of food allergy, Frossard et al. showed that exogenous supplementation of IL-10 decreased food-induced anaphylaxis and prevented IgE-type sensitisation to common food allergens.27 Taken together, these data support the finding that a reduction of serum IL-10 levels is related to the occurrence and development of pollen-related food allergies.
The most interesting finding of our study was that the serum TGF-β levels in pollinosis sufferers with a food allergy were abnormally elevated, and the highest levels of TGF-β appeared in the group with the most serious symptoms. Previous studies have presented controversial conclusions with respect to the role of TGF-β in anaphylaxis. Okamoto et al. found that oral administration of high dose TGF-β can suppress a food-induced IgE response and systemic anaphylaxis,28 whereas an earlier study conducted by Kim et al. showed that TGF-β was key for activating mast cells and the development of anaphylaxis.29 Therefore, it is likely that an optimal level of TGF-β may be beneficial, whereas high and low levels could be harmful. However, TGF-β could also be pathogenic in some inflammatory diseases, such as autoimmune diseases and colitis, when combined with pro-inflammatory cytokines (e.g., IL-6 or IL-23) by promoting the generation of Th17 cells.30,31 The combination of TGF-β and IL-6 can also prevent the generation of Foxp3 cells,32 which might explain why higher serum TGF-β levels were associated with more severe food allergy symptoms. In addition, IL-23 can restrain regulatory T cell activity and suppress IL-10 function, thereby breaking the immunological homeostasis of the gut.33,34 Thus, further assessment of IL-6 or IL-23 levels might help us better understand the pathogenesis of food pollen allergies.
This was a preliminary study with some limitations. First, the study sample was relatively small. Second, patients allergic to different pollens in different seasons were recruited, and therefore only a few people were monosensitised to only one type of pollen. However, we carefully compared the serum levels of IL-10 and TGF-β among patients allergic to different types of pollen and between patients enrolled during different allergy seasons, and no differences were found. Thus, the results of our study were objective and convincing.
In summary, our data indicate that with the exception of a defect in regulatory cells represented by the reduction of IL-10, there may be potential intrinsic mechanisms (e.g., Th17 or IL-23 together with TGF-β) that are involved in the development of pollen food allergies.
Ethical disclosuresProtection of human subjects and animals in researchProtection of human and animal subjects. The authors declare that no experiments were performed on humans or animals for this investigation.
Patients’ data protectionConfidentiality of data. The authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentRight to privacy and informed consent. The authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.
Conflict of interestsThe authors have no conflict of interests.