INTRODUCTION
In Spain, the pediatric vaccination schedule includes two MMR doses in all children (first one at the age of 15 months and a second dose at four years old), except those in whom there is contraindication. Contraindications include patients with immunodeficiency or immunossupression, resulting from leukemia, lymphoma, generalized malignancy, immune deficiency disease, or immunosuppressive therapy, and should not be vaccinated. Never, if there is moderate or severe acute illness, should patients be vaccinated until the illness has improved. Receipt of antibody-containing blood products may interfere with seroconversion to MMR vaccination. Vaccine should be given 2 weeks before or deferred for at least 3 months following administration of an antibody-containing product1-3.
Since the measles and mumps components used in MMR vaccine are grown in cultures of fibroblast from chick embryos, for a long time there have been concerns about the presence of egg protein in the vaccine and the recommendations given to egg allergic patients. Different studies have focused on determining the amount of egg proteins in MMR vaccines, finding from any proteins, picograms valuation or between 0.5-1 nanogram of ovalbumin per 0.5 ml dose4,5. This may be due to different methods used to measure egg protein or differences between batches of the vaccine.
Two MMR vaccines are available in Spain: Vacuna Triple MSD (Sanofi Pasteur MSD) and Priorix (GSK). Triviraten vaccine (Berna), grown in diploid cells, was available until 2004 offering an alternative in egg allergic patients. Moruviraten vaccine (Berna Biotech) is the only available vaccine not grown in chick embryo fibroblasts but doesn't include a mumps strain.
Some allergy clinics have experience in vaccinating egg allergic patients using regular MMR vaccines. However there has not been an immunological approach to study the recognition of this vaccine available in Spain by allergic patients.
The aim of this study was to evaluate the clinical safety of a conventional MMR vaccine in a population of egg allergic patients and to determine the presence of egg allergens in a conventional MMR vaccine and if IgE antibodies from egg allergic can recognize egg allergens in this vaccine.
MATERIALS AND METHODS
Patients
Patients were recruited from a population of children referred to our allergy clinic with the suspected diagnosis of egg allergy (From January 2005 until May 2006). Those children 15 months old with a confirmed diagnosed of egg allergy were included.
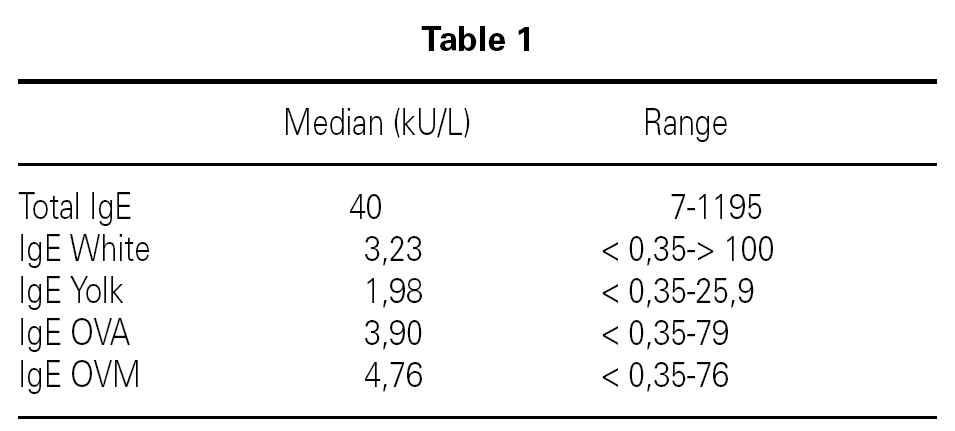
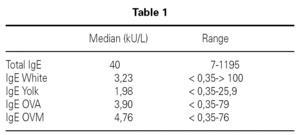
Inclusion criteria were: Confirmed egg allergy diagnosis by means of skin prick test (positive: wheal > 3 mm) using commercial extracts of egg white, yolk, ovoalbumin and ovomucoid (Bial-Aristegui laboratories) and or positive specific IgE antibodies measurement (> 0.35 KU/l) (CAP System Pharmacia FEIA) to same allergens. Informed consent from parent was obtained.
Exclusion criteria: patients with adrenaline use contraindicated.
Sera of egg allergic patients were used for the immunological study.
Vaccine administration
In all patients, a skin a prick test with non diluted MMR vaccine (Priorix, GSK) was made. (Histamine, 10 mg/ml, and physiological saline were used as positive and negative control respectively). If negative, each patient received a single dose of measles, mumps, rubella (MMR) vaccine. If positive, a fractionated injection of the vaccine was made according the SEICAP recommendations (2004)2:
0.05 non-diluted
0.10 non-diluted
0.15 non-diluted
0.20 non-diluted
Waiting 20 minutes between every dose
In all cases the vaccine was administered in Allergy Department of Ramon y Cajal Hospital with an observation period of 30 minutes after the shot.
Immunological study
SDS-PAGE and SDS-PAGE immunoblotting: Prorix (GSK) Vaccine proteins were separated by Sodium dodecylsulfate-polyacrylamide gel electrophoresis (SDS-PAGE) according to the discontinuous method described by Laemmli in 12.5 % polyacrylamide gel, with parallel application of a reference standard of proteins with known molecular masses. 20 g proteins, according to Bradford assay, were applied per lane. To prepare the samples under reducing conditions, they were dissolved in 0.125 M HCl-Tris buffer, pH 6.8, and were dissociated with SDS and 5 % ?-mercaptoethanol at 100 °C for 5 min.
After electrophoresis, gels were stained in 0.1 % Coomassie Brilliant Blue R-250 dissolved in methanol/acetic/distilled water (4: 1: 5).
Separated protein bands by means of electrophoresis were transferred to polyvinylidene difluoride membranes (Immobilon-P, Millipore, Milford, MA). Immunodetection was performed using a chemiluminescent detection reagent (Lumigen PS-3) following the manufacturer's instructions (ECL + Plus Western blotting detection reagents. Amersham Pharmacia Biotech, Amersham, UK).
Membranes were immunolabeled with two different groups of patients. Patients older than 15 months who have not outgrown their egg allergy (challenge test positive) and patients, included in this study, who received regular Triple Viral vaccine (Prorix-GSK)
RESULTS
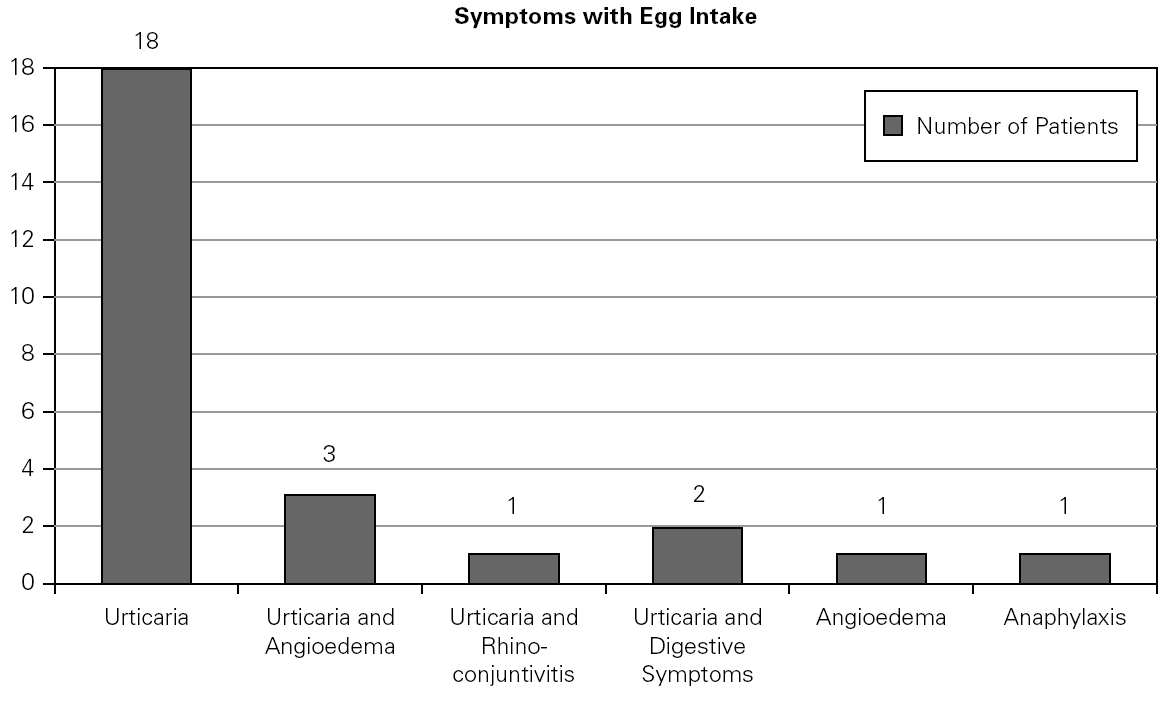
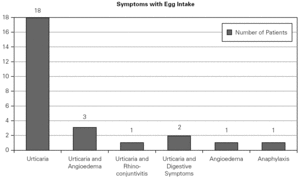
A total number of 26 patients (18 males) were included presenting with different symptoms after egg intake (fig. 1) and different specific IgE levels. (table 1). All patients have safely received MMR vaccine in a single-dose at our Department. None of them had a positive SPT with the vaccine. Every patient received a full dose. No immediate or delayed reaction (neither local nor systemic) was found in any of the patients.
Figure 1.--Clinical manifestations of patients.
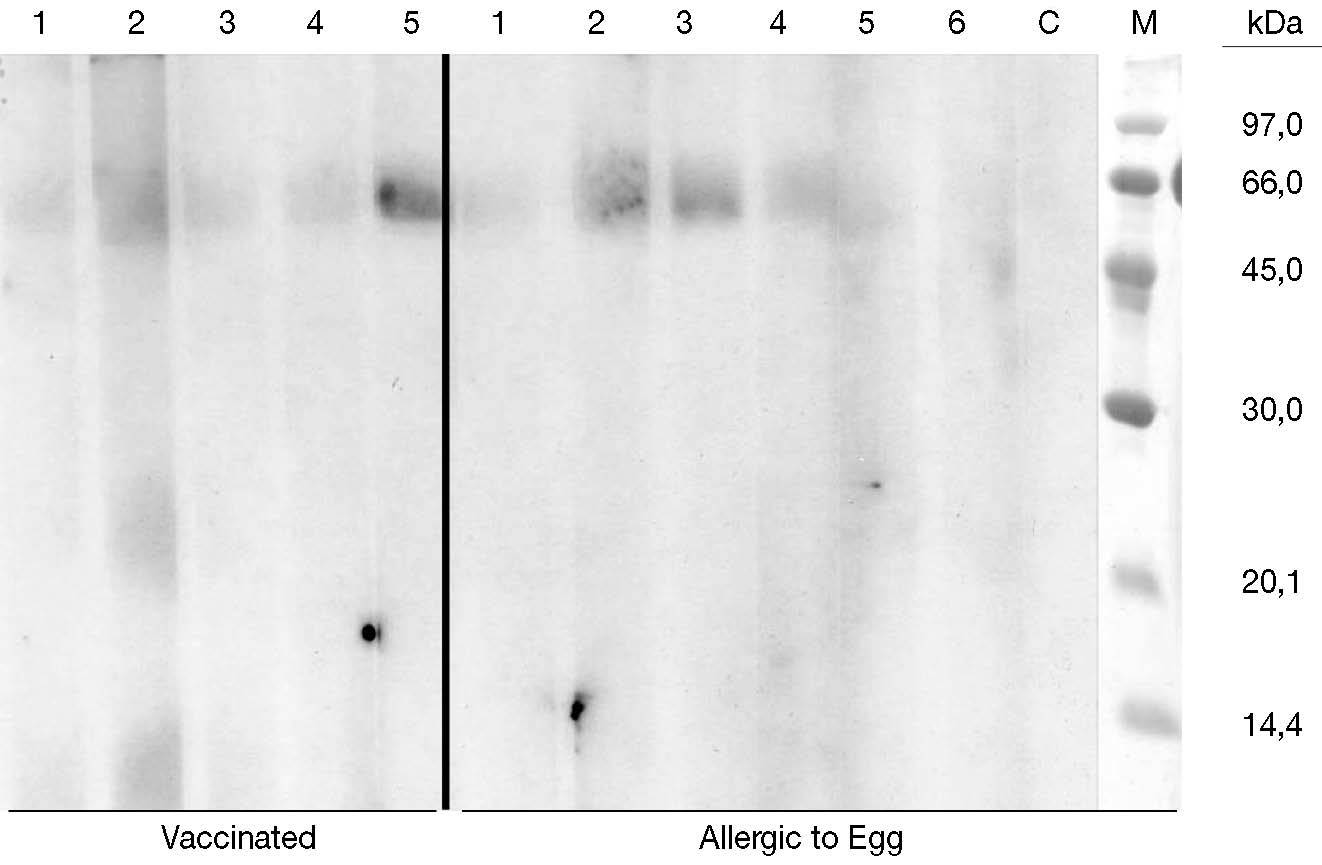
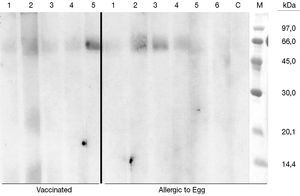
Five sera of vaccinated patients and 6 control sera of egg allergic patients (positive oral challenge) were finally used to immunolabel the membranes. No binding bands corresponding to egg proteins were found in any of the patients (fig. 2). In some of the lanes we obtain a band around 60 KDa that probably corresponds to human albumin (used in the vaccine).
Figure 3.--Two groups of patient sera were used for labelling Immunoblot membranes. Left part of the figure corresponding to vaccinated patients (lane number 5 corresponds to the only patient included presenting with anaphylaxis after egg intake). Right part (lane 1 to 6) shows egg allergic patients, non tolerant, followed at Ramon y Cajal Allergic Clinic. Lace C is negative control (non atopic patients) and M lane represents the pattern of molecular masses.
DISCUSSION
Different vaccines (influenza, measles-mumps-rubella (MMR), rabies and yellow fever) may contain egg protein. It is important when evaluating the risk of reaction to consider both the production methods of these vaccines and the patient's clinical history. While inactivated influenza vaccine, for example, is grown in the chorioallantoic fluid or ovalbumin of chick embryos and the allergenicity of these vaccines varies with methods of preparation (with whole virus from red cell eluates containing more chick egg protein than centrifuged or chemically precipitated vaccines), the measles component of MMR is grown in chick fibroblast cultures that do not contain egg antigens. It is very important, if egg allergy is suspected, to refer the patient to an allergist in order to confirm a positive clinical history.
Since many years ago different groups have focused on the safety of these vaccines in allergic patients. One of the first reports of MMR vaccine was in 1963. Kamin et al.6,7 reported the safe administration of a measles vaccine to 22 children with confirmed egg allergy (by food challenge). Another group reported the safe administration of a measles vaccine to a child allergic to eggs.8 During the 1980s, Miller et al.9 and Greenberg and Birx10 administered the MMR vaccine to a total of 19 children who were allergic to eggs without any reported reaction. More recently, Kemp et al.11 successfully immunized 32 children who were allergic to eggs, without any adverse reactions. Lavi et al.12 safely immunized 90 such children using one dose MMR vaccine. Finally, investigators from Italy administered the measles vaccine safely to 23 children with severe egg allergy confirmed by positive open food challenges.13 Subsequently, the same group has safely immunized an additional 60 children with allergy to eggs.14 Other studies (Fasano et al, Aikin et al and Freigang et al15-18) had reported adverse reactions, including anaphylaxis, after MMR administration in patients allergic to egg proteins but not all of these patients had a confirmed diagnosis and in some cases other components of the vaccine such as gelatin19 or neomycin have been implicated. Skin testing for reactions to the vaccine lacks specificity and sensitivity16. Our experience agrees with these previous studies in not observing any reaction in 26 vaccinated patients with confirmed diagnosis of IgE mediated egg allergy.
There are few approaches to determine the presence of egg allergens in MMR vaccines. Khakoo et al20 reviewed the evidence for egg as the agent responsible for allergic reactions to MMR or measles vaccine, concluding that the amount of ovalbumin in the vaccine is so small that is highly unlikely that it could cause reactions in allergic individuals and also points out that other allergens than egg may be responsible (neomycin and gelatin) and few reports have focused on them. We have performed an immunological assay with Vacuna Triple MSD (Sanofi Pasteur MSD) and Priorix (GSK). No binding bands corresponding to egg proteins were found in any of the patients.
Negative results found in SPT support the absence of clinical reaction against components and immunological studies show that there is no detectable amount of egg protein in this vaccine to produce an IgE mediated reaction.
In the last two years different Spanish21-27 groups have reported their experience using MMR vaccine in egg allergic patients. As a result of these studies, there has been an immediate change in the recommendations of The Food Allergy Committee of Spanish Society of Clinical Immunology and Pediatric Allergy (SEICAP). Since February 2006 the vaccination of egg allergic patients with any of the two regular MMR vaccines available is recommended. Only those patients presenting with anaphylactic reaction after egg ingestion should be referred to a hospital.
With this immunological study and the previous clinical experience reported we can conclude that MMR can be safely administrated in children allergic to egg.
Correspondence:
I. Cerecedo Carballlo
Servicio de Alergia
Hospital Ramón y Cajal
Crta Colmenar Viejo Km 9.100
28034 Madrid
Tel: 913368341
Fax: 913368693