INTRODUCTION
Atopic dermatitis is a chronic, pruriginous, reactional and recidivating dermatosis most frequently presented among infants and young children. With large worldwide variability, the frequency of atopic dermatitis ranges from 2 % to 30 %1. Genetic and environmental factors as well as allergen exposure have been identified as risk factors of atopic dermatitis2. More specifically, the early introduction of supplemental feedings as well as the diversity of the diet (feeding 4 or more different food groups) appears to increase the risk of atopic dermatitis3. In addition, early weaning has been reported in 15-40 % of children with atopic dermatitis4, and infants with AD may have a high prevalence of food sensitization5.
However, a definitive role of food hypersensitivity as a causal or promoting factor of atopic dermatitis is not yet definitively established6,7. We therefore designed the present study in order to investigate the relationship between hypersensitivity to cow's milk, hen egg, wheat, fish, soy, or legumes and atopic dermatitis in young children. Cow's milk, hen egg, wheat, soy and peanuts are among the most common foods with allergenic potential in childhood8,9. As secondary study outcomes, positive family history of allergies and timing of weaning were evaluated as potential contributors of the risk of atopic dermatitis.
MATERIAL AND METHODS
The study consisted of two phases. The first phase was designed as a case-control study. The second phase was a reevaluation performed 4 years later. The study was approved by the Research and Ethics Committees at the Hospital Infantil de México "Federico Gómez" (HIM) and parental written informed consent was obtained from participants.
We identified 32 potentially eligible children of ≤ 3 years of age who had a presumptive diagnosis of atopic dermatitis. The diagnosis was confirmed according to the criteria established by Hanifin and Rajka10. The control group consisted of children receiving orthopedic treatment at the HIM, without any neoplasic, pulmonary, or infectious diseases, and not taking any medication chronically. Control children were carefully examined to rule out any clinical evidence of atopic dermatitis.
Information about duration of breastfeeding, the age at which infants were weaned with different foods, family history of asthma, allergic rhinitis, atopic dermatitis and urticaria, were obtained from the mothers. Food hypersensitivity was evaluated by skin prick test and patch test.
Skin prick test for cow's milk, hen egg (yolk and white), wheat, fish, soy, and legumes was performed as follows. Briefly, one drop (50 μl) of food glycerin extracts (Nelco Laboratories, New York, NY, USA), one drop of histamine as positive control, and one drop of glycerin as negative control were placed on the skin surface of their backs. An instant pressure by means of a sting positioned at 90° was performed to the skin containing the drops. Skin response was evaluated according to Bock and May's criteria11,12. The presence of wheal and erythema was evaluated 15-20 min after the allergen challenge. An indurated wheal of a diameter of at least 3 mm was considered as a positive test of food hypersensitivity.
Patch test was performed as previously described elsewhere13. Briefly, one drop (50 μl) of each cow's milk (containing 3.5 % fat), hen egg (yolk and white), wheat powder (1 g/10 mL of water), and soy were placed in a filter paper with 12 mm aluminum cups (Finn Chambers on Scannportape, Hermal, Reinbeck, Germany) which was applied on a skin surface non involved of dermatitis of the children's back. The occlusion time was 48 hours, and skin reaction was evaluated at 20 min and 24 hours after removal of the cups.
Statistical analysis. Independent proportions of study outcomes were compared between cases and controls by means of the z test. For those proportions that were significantly different (P < 0.05), odds ratio (OR) and its corresponding 95 % confidence interval were obtained by standard procedures
RESULTS
Cases and controls were comparable in their demographic characteristics (table 1). There was no difference between groups in the prevalence of either positive skin prick test or positive patch test for food hypersensitivity. However, when the diagnostic criteria for food hypersensitivity was extended to a positive result with any of the two skin tests (either skin prick or patch test), significant differences between cases and controls were observed (table 1), with an OR of 4.2 (95 %CI 1.3 to 13.4). The highest prevalence of positive prick test among cases was for hen egg and legumes, followed by cow's milk, fish and soy whereas among controls it was for legumes and fish (table 2). The highest prevalence of positive patch tests among both cases and controls was for hen egg. However, due to the small number of cases having specific food allergies, we did not perform any statistical comparison between cases and controls regarding these differences.
No significant difference of family history of allergic diseases (asthma, allergic rhinitis, atopic dermatitis and urticaria) was observed between cases and controls (table 1). Similarly, approximately 90 % of children in the two groups were weaned at ≤ 6 mo. of age.
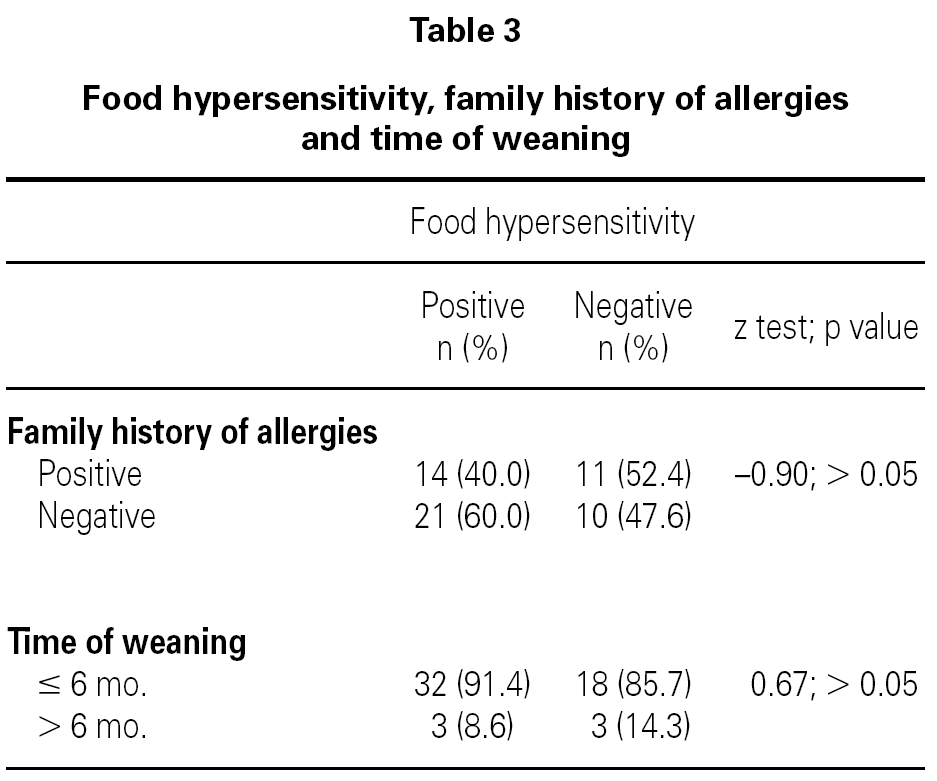
In a post-hoc analysis, children who had food hypersensitivity were compared to children without it, independently of the presence or absence of AD. We did not find any difference between groups in relation to children with family history of allergies or weaned at ≤ 6 months of age (table 3).
In the second phase of the study, 4 years later, we were able to reinvestigate the state of AD in 13 (46.4 %) infants out of 28 cases. Of them, 11 followed an exclusion diet and 6 (46.1 %) continued with dermatitis. Of the 29 controls, we obtained information from 15 (51.7 %) infants. Of them, only one case developed atopic dermatitis.
DISCUSSION
In our study, the overall of positive prick test for food hypersensitivity varied from approximately 4 % to 25 % among young children with food hypersensitivity. The rate was higher with patch test, varying from approximately 30 % to 70 %. In comparison to control children, an OR of 4.2 (95 %CI 1.3 to 13.4) in either a prick or patch positive test was observed in children with AD. Our results are in agreement with previous reports showing that a large proportion of children and infants with AD also have food hypersensitivities or food allergies2,5.
A high rate (42 %) of first-degree relatives of children with AD may also have the disease13. Similar rates were observed in our study: approximately 46 % of children with atopic dermatitis had parents with an allergic disease. However, the control group also had a high rate (approximately 43 %) of parents suffering from allergic diseases.
In Mexico, the maternity period is typically limited to 45 days postpartum. This may explain the 90 % rate of children weaned at ≤ 6 months. However, this is not an isolated example. A British study showed that the median age at introduction of solid food was 11 weeks14. In addition, our study failed to identify weaning ≤ 6 months as a risk factor of atopic dermatitis. Similarly, no relationship between early weaning and atopic dermatitis was recently found in a European study cohort15, and another study suggested that the early introduction of solids might be less harmful than was initially though16. In contrast, there are current European and American and recommendations to exclusively breastfeed babies for at least 4-6 months3. Indeed, our study cannot suggest any modification to these recommendations solely based on our results. However, our findings may help in counseling women who cannot exclusively breastfeed their babies for different reasons and who should be reassured that there is some evidence that early weaning may not increase the risk of atopy in their infants.
In conclusion, the present study found a higher risk of atopic dermatitis among young children who had hypersensitivity to cow's milk, hen egg, wheat, fish, soy, or legumes. Positive family history of allergies and early weaning were not found to be relevant risk factors.
ACKNOWLEDGEMENTS
The authors are grateful to Ms. Olivia Tischler for editing the manuscript.
Correspondence:
Elizabeth Estrada-Reyes, MD
Consulta de Alergia Pediátrica
Torre Diamante, Apt. 300
Hospital Ángeles Metropolitano, Tlacotalpan No. 59
México DF 06760, México
E-mail: elizabeth.estradareyes@gmail.com