INTRODUCTION
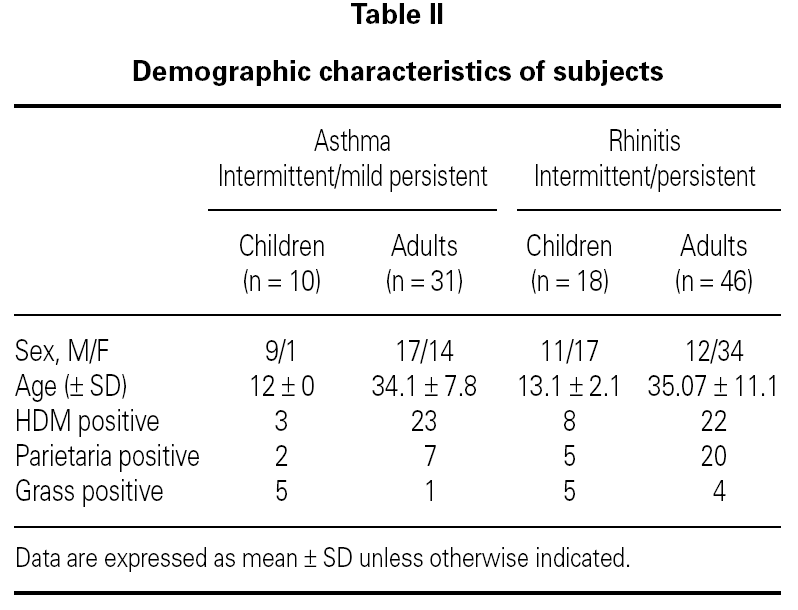
The role of sublingual immunotherapy (SLIT) as a viable alternative to subcutaneous immunotherapy is based on well-documented experimental evidence, as noted in the consensus statement of the World Health Organization and in the new ARIA position paper 1-2. The safety and the good tolerability of sublingual immunotherapy (SLIT) has already been proved in large population trials carried out in allergic patients 3-6, but so far only one study have investigated the occurrence of immediate adverse reactions in allergic patients after a 2-hour ultra-rush regimen of SLIT 7. The aim of the present study was to evaluate the safety and the tolerability of a very fast (20 minutes) ultra-rush regimen of a chemically modified allergen extract (sublingual allergoid SLIT), as an alternative to the conventional several weeks incremental schedule (table I).
MATERIAL AND METHODS
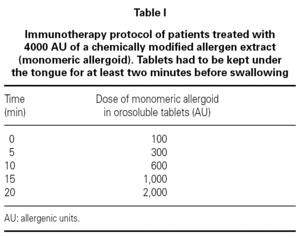
We studied 28 children (20 male, mean age 13,3 ± 2,1 yr) and 77 adults (29 male, mean age 34,7 ± 9,9 yr) with a history of intermittent/persistent rhinitis or intermittent/mild persistent asthma due to House Dust Mite (n = 56), Parietaria (n = 34) and Timothy-grass (n = 15) (table II). All the patients (or parents of children) freely gave their informed consent. The diagnosis of allergic rhinitis was done at the study entry according to ARIA position paper 2, and the diagnosis of asthma and the assessment of its severity were done according to Global Initiative for Asthma 2003 8. Atopic status was assessed by skin prick tests carried out with a standard panel of commercial extracts (Lofarma S.p.A, Milan, Italy). The panel included: Dermatophagoides pteronyssinus, grass pollen, Parietaria judaica, Phleum pratense, Artemisia vulgaris, olive, dog and cat dander, Alternaria, and Cladosporium plus a positive (histamine 1 %) and a negative (isotonic saline) control.
Only patients with a positive skin prick test, i.e. those with a wheal mean size > 3 mm 9, and specific IgE, titrated by CAP System® (Pharmacia-Upjohn), were included in the study. All grass pollen sensitive patients were submitted to rush therapy protocol out of pollen season.
The patients were prescribed a commercial SLIT with a monomeric allergoid in orosoluble tablets (Lais®, Lofarma SpA, Milan, Italy). The product was titrated in allergenic units (AU) and standardized against the in-house reference preparation. In the present study, the starting dose (100 AU) was several times higher than that recommended in the standard schedule (25 AU). After the last dose of ultra-rush SLIT schedule, patients were kept under observation for 3 hours. All patients were instructed to record the presence and severity of respiratory and nasal symptoms on a diary card covering 14-consecutive-day period after the ultra-rush protocol. The symptoms considered were: sneezing, nasal itching and obstruction, rhinorrhea, lacrimation, conjunctival itching and hyperemia, cough, wheezing, and chest tightness. In case of appreciable oral or systemic reactions occurring at home, patients were told to take a tablet of cetirizine and immediately consult their allergologist. All patients were instructed to continue with the maintenance doses recommended by the manufacturer.
RESULTS
All patients tolerated the treatment very well. Only one patient out of 105 (0.9 %) had a mild local symptom (gastric pirosis) that occurred 30 minutes after the last initial dose and spontaneously disappeared within one hour. This study validates the concept that high-dose allergen SLIT with an ultra-rush regimen is safe and well tolerated, with very low occurrence of mild adverse effects. The ultra-rush regimen will allow clinicians to propose immediate maintenance treatment even to patients arriving when their pollen-induced symptoms were already present. In the similar previous study 7, it was observed a substantial lack of adverse reactions in 91 allergic patients treated by an ultra-rush SLIT regimen with either native or chemically modified allergens, during a 2-hour up-dosing period with increasing doses every twenty minutes.
DISCUSSION
In the present study, the cumulative dose after 20 minutes was several times the dose administered during the conventional schedule. Even so, most patients suffered no increase in the adverse event rate as we observed only one mild adverse reaction involving the gastro-intestinal tract. This occurred 30 minutes after the last initial dose and spontaneously disappeared within one hour, suggesting that an observation period of three hours should be sufficient. As previously shown by Lombardi 4, the sublingual monomeric allergoid proved to be safe and well tolerated. In fact, in our study, only one out of the 105 patients reported side-effects during the treatment.
One of the main implications of our clinical observations is that with the ultra-rush SLIT protocol, adverse reactions seem paradoxically to be less than in other conventional SLIT protocol studies. We can conclude that, as a whole, the safety and tolerability profile of this ultra-rush SLIT schedule is very encouraging. Moreover, it was well accepted by patients since it dramatically simplifies and shortens the SLIT initial phase.