Sensitisation to home aeroallergens (mites, furry animals and cockroaches) is predominant in patients in our and other areas. Covariation of sensitisation (CS) to these allergens could be due to cross-reactivity or parallel sensitisation.
MethodsSkin prick tests were performed to common and second-line home aeroallergens, shrimp and tropomyosin in 253 paediatric patients seen in our Unit due to chronic respiratory symptoms. CS among the main allergens was analysed by means of Cohen's kappa coefficient of concordance (κ).
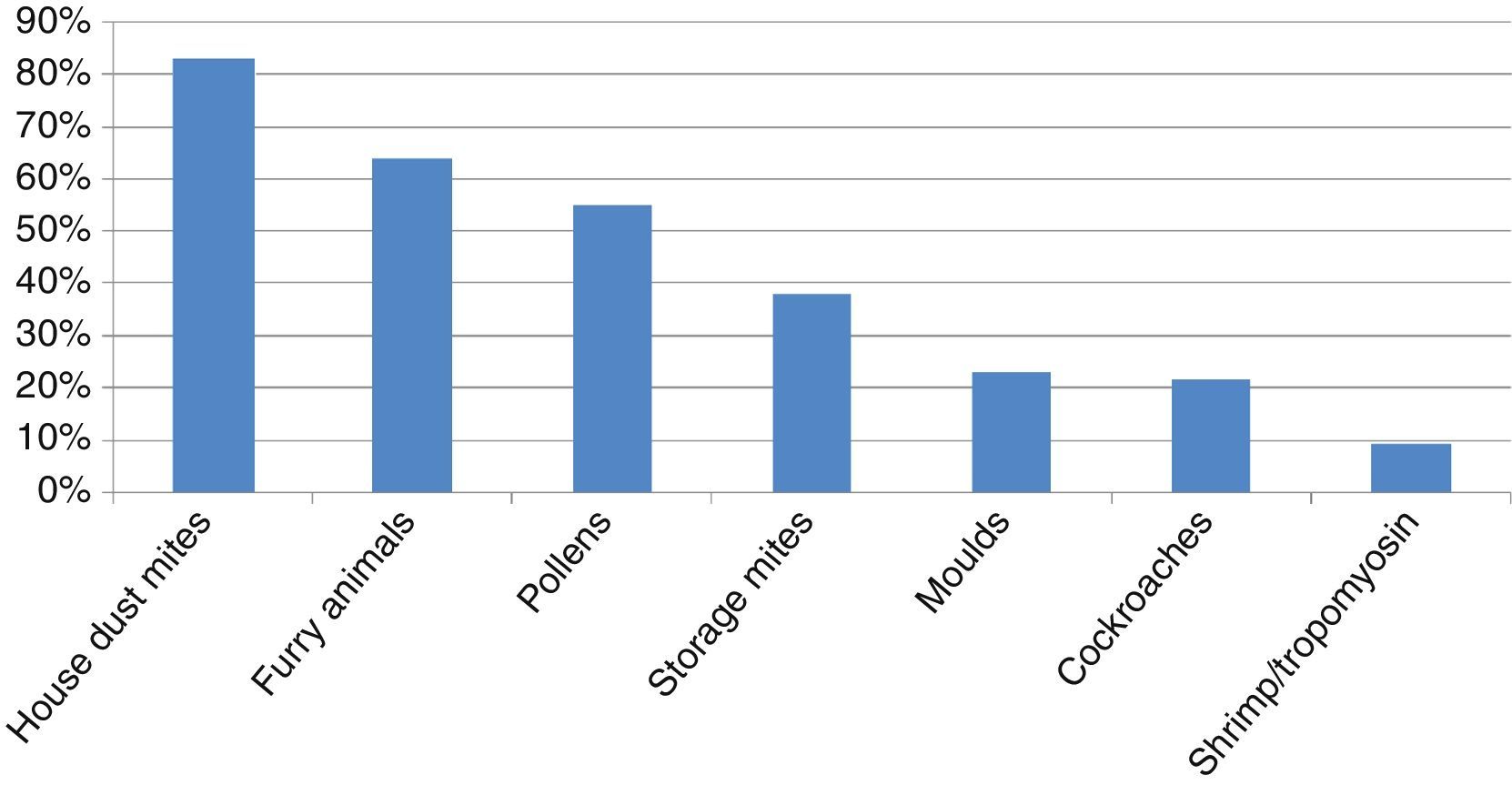
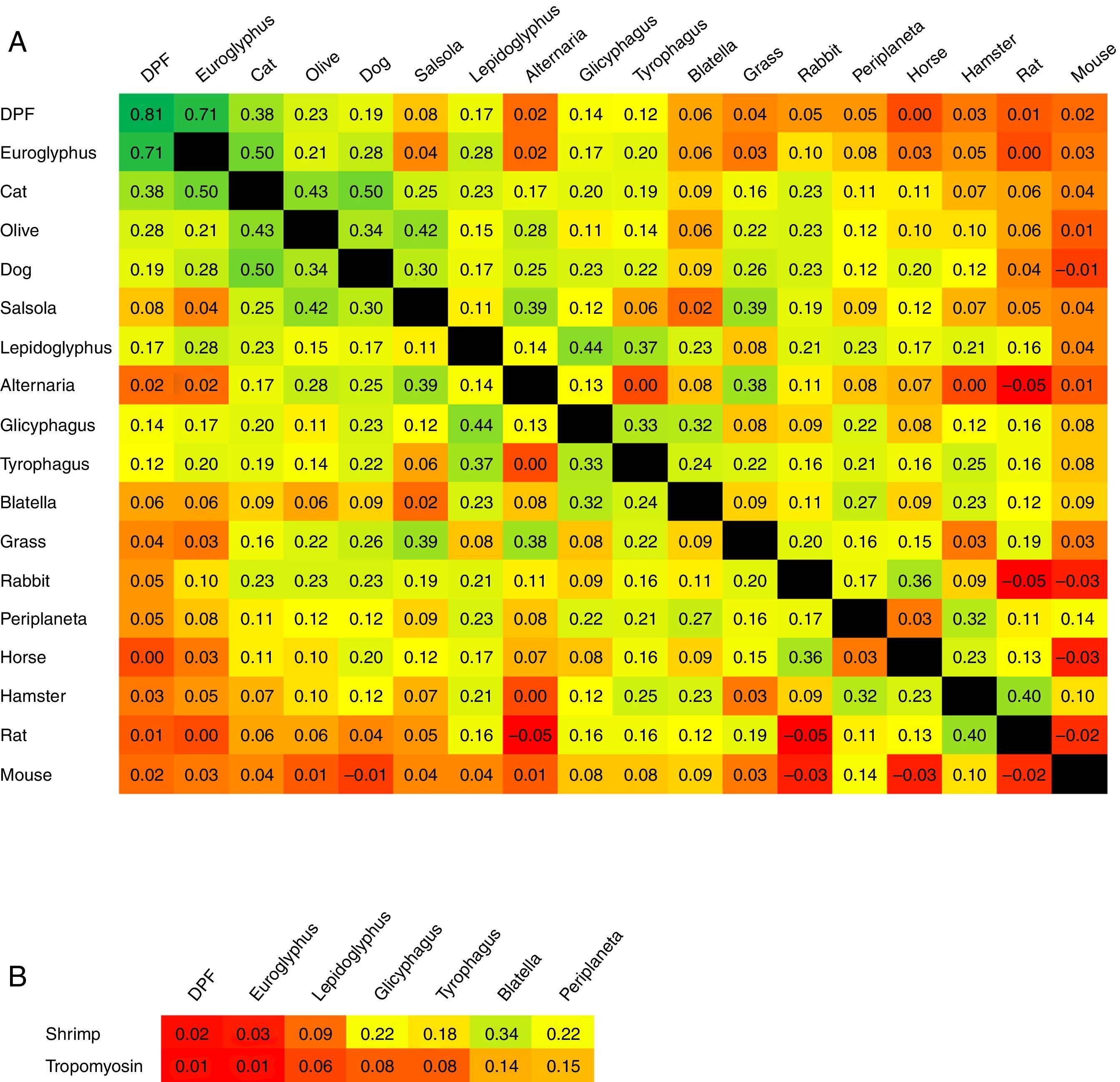
ResultsHouse dust mites (HDM) were the main allergens involved in sensitisation, followed by furry animals, pollens, storage mites (SM), moulds and cockroaches. CS was very good between D. pteronyssinus and D. farinae (κ=0.81), and good between Dermatophagoides and Euroglyphus (κ=0.71), and decreased markedly to poor (κ<0.20) between HDM and SM or cockroaches. CS among SM and cockroaches was moderate to fair (κ=0.21–0.44). CS was good between shrimp and tropomyosin (κ=0.62), but null between shrimp-tropomyosin and HDM (κ=0.01–0.02), and poor to fair between shrimp-tropomyosin and SM or cockroaches (κ=0.06–0.34). CS between cat and dog was moderate (κ=0.50).
ConclusionsWe have shown the usefulness of κ in exploring CS in a population. In our area, with high sensitisation to HDM and other home allergens, CS is limited for most pairs of allergens, pointing to a lower incidence of cross-reactivity than could be expected, especially between HDM and SM or other invertebrates.
Animal-derived aeroallergens (mites, furry animals and cockroaches) are predominantly, but not exclusively, home allergens in childhood. Mites are a major source of sensitisation worldwide, especially in regions where the humidity and temperature conditions favour their development.1 Currently, house dust mites (HDM) are the most abundant mites in indoor habitats, and thus the primary source of sensitising allergens. However, other mite species commonly known as storage mites (SM) are increasingly regarded as important indoor allergens.2 Furry animals, in particular cats and dogs, are also among the most important sources of indoor allergens.3,4 Exposure to allergens from other furry animals such as horses, rabbits, hamsters or other rodents has also been associated to allergic sensitisation. Cockroaches represent another common source of indoor allergens worldwide.5 There are large geographical differences in the presence of mites, furry animals and cockroaches in the habitats of children all over the world, and even within the same country; accordingly, there is variance in the prevalence of sensitisation to these allergens.
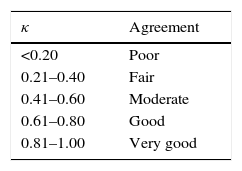
Allergic polysensitisation, including simultaneous sensitisation to several animal-derived home aeroallergens (ADHA) is a common phenomenon in atopic subjects.6 Simultaneous sensitisation to two or more allergens in a frequency higher than expected by chance, sometimes referred to as covariation of sensitisation (CS), may originate from cross-reactivity (when an antibody originally generated against one allergen binds to a similar allergen from another source) or be caused by parallel sensitisation (primary cosensitisation to different allergens).7,8 Covariation of sensitisation could be objectively graded by means of Cohen's kappa coefficient (κ), a statistic which measures the agreement between qualitative (categorical) variables (allergen sensitisation in our case) when agreement occurring by chance is taken into account.9 Concordance measured by κ can adopt values between 1 (total agreement) and 0 (total absence of agreement other than by chance), and even negative values. A high κ (0.80 or above) suggests very high CS, whether due to parallel sensitisation, cross-reactivity or both. In contrast, a low κ (0.20 or below) points to very low or absent CS and implies negligible cross-reactivity.
Cross-reactivity between Dermatophagoides pteronyssinus and D. farinae is well known. Moderately high cross-reactivity has been reported between Euroglyphus maynei and Dermatophagoides species.10,11 The problem of cross-reactivity between HDM and SM or between the different SM species has been studied.12 Variations may be related to actual exposure to SM. Thus, in areas with high exposure to these two groups of mites, SM may be a primary sensitising agent.2 House dust mite allergens have also been proposed to cross-react with allergens from other invertebrates, including insects, molluscs, and crustaceans.7 Cross-reactivity is also recognised among furry animals3 or cockroaches.5 Some protein families can be responsible for cross-reactivity among mites, cockroaches and crustaceans, especially tropomyosin.8 All these aspects make interpretation of the skin prick test (SPT) and specific IgE (sIgE) results difficult.
Patients seen in our hospital live in a warm coastal metropolitan area where mites are the main sensitisers. We have explored the sensitisation profile of children seen in our Pediatric Allergy Unit with persistent or recurrent symptoms suggestive of respiratory allergy. For this purpose, the usual panel of aeroallergens was supplemented with an expanded panel of second-line ADHA. The aim of this work was (A): to define the importance of second-line ADHA sensitisation in children in our area; and (B): to analyse the concordance of sensitisation to different allergens by means of κ, as an estimate of CS among them.
Materials and methodsPatientsThe study sample was extracted from all consecutive patients attending our Pediatric Allergy Unit over most of the autumn–winter season of 2014–2015. Patients who were skin-prick tested with our battery of common aeroallergens as a part of their evaluation of chronic respiratory symptoms (rhinitis, cough, wheezing, etc.) were included in the study.
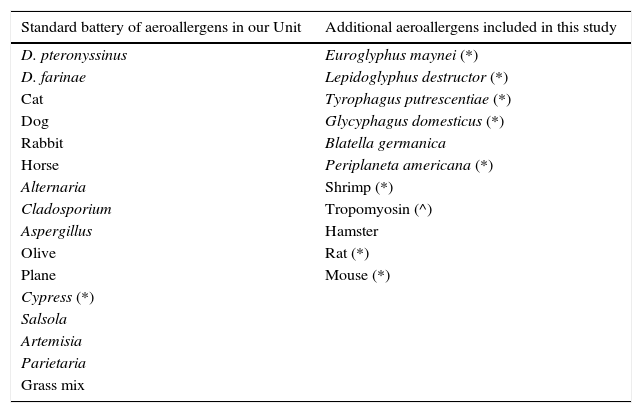
Skin testsThe battery of common aeroallergens for skin testing is shown in Table 1, together with the additional aeroallergens tested during the study period. Prick tests were performed with commercial allergen extracts available in our hospital, according to international recommendations.13 Allergen sensitisation was defined as a skin prick test reaction with a mean wheal diameter of ≥3mm.
Aeroallergens tested in subjects in our study.
| Standard battery of aeroallergens in our Unit | Additional aeroallergens included in this study |
|---|---|
| D. pteronyssinus | Euroglyphus maynei (*) |
| D. farinae | Lepidoglyphus destructor (*) |
| Cat | Tyrophagus putrescentiae (*) |
| Dog | Glycyphagus domesticus (*) |
| Rabbit | Blatella germanica |
| Horse | Periplaneta americana (*) |
| Alternaria | Shrimp (*) |
| Cladosporium | Tropomyosin (^) |
| Aspergillus | Hamster |
| Olive | Rat (*) |
| Plane | Mouse (*) |
| Cypress (*) | |
| Salsola | |
| Artemisia | |
| Parietaria | |
| Grass mix |
Commercial extracts from ALK-Abelló, Madrid, Spain; except (*) from Bial-Aristegui, Bilbao, Spain and (^) from Laboratorios LETI S.L., Madrid, Spain.
Data from patients and results of skin tests were transferred to SPSS (v17.0 SPSS, Chicago, IL, USA). The descriptive analysis included frequencies and percentages for categorical variables, and medians for numerical variables with a non-normal distribution. Covariation of sensitisation for each pair of allergens was estimated by means of κ and interpreted using the Altman scale (Table 2).14
EthicsOur work was performed under usual clinical practice conditions and was approved by the ethics committee for clinical investigation of our hospital.
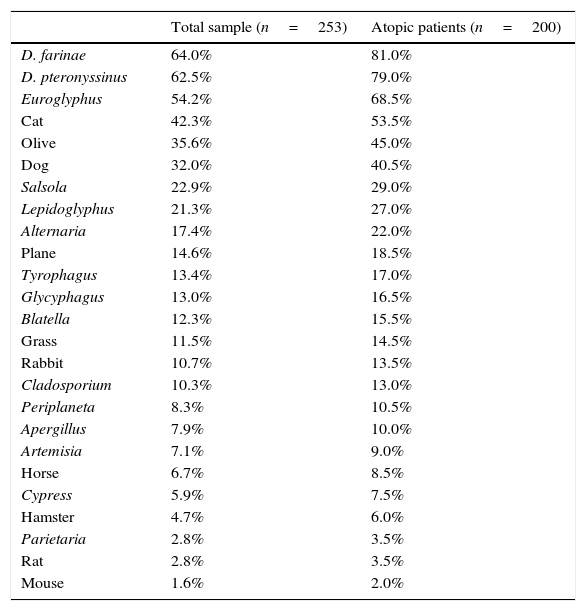
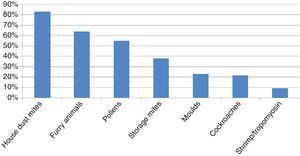
ResultsA total of 253 subjects were included in the study, of which 67% were males. The age ranged from 0 to 16 years (96% were 2–14 years-old; median 8 years). A total of 91% of the patients were diagnosed with asthma, rhinitis or both (57% with asthma, 66% with rhinitis), and 200 (79%) were sensitised to one or more of the allergens tested, and were regarded as atopic individuals. The frequency of sensitisation to the main groups of allergens in the atopic patients is shown in Fig. 1. The frequency of sensitisation to each of the individual aeroallergens tested is shown in Table 3. Only 21 subjects were monosensitised (10.5% of those with atopy), mainly to D. pteronyssinus–D. farinae (8 subjects) or Alternaria (4 subjects).
Frequency of sensitisation to the aeroallergens tested among total sample and atopic patients.
| Total sample (n=253) | Atopic patients (n=200) | |
|---|---|---|
| D. farinae | 64.0% | 81.0% |
| D. pteronyssinus | 62.5% | 79.0% |
| Euroglyphus | 54.2% | 68.5% |
| Cat | 42.3% | 53.5% |
| Olive | 35.6% | 45.0% |
| Dog | 32.0% | 40.5% |
| Salsola | 22.9% | 29.0% |
| Lepidoglyphus | 21.3% | 27.0% |
| Alternaria | 17.4% | 22.0% |
| Plane | 14.6% | 18.5% |
| Tyrophagus | 13.4% | 17.0% |
| Glycyphagus | 13.0% | 16.5% |
| Blatella | 12.3% | 15.5% |
| Grass | 11.5% | 14.5% |
| Rabbit | 10.7% | 13.5% |
| Cladosporium | 10.3% | 13.0% |
| Periplaneta | 8.3% | 10.5% |
| Apergillus | 7.9% | 10.0% |
| Artemisia | 7.1% | 9.0% |
| Horse | 6.7% | 8.5% |
| Cypress | 5.9% | 7.5% |
| Hamster | 4.7% | 6.0% |
| Parietaria | 2.8% | 3.5% |
| Rat | 2.8% | 3.5% |
| Mouse | 1.6% | 2.0% |
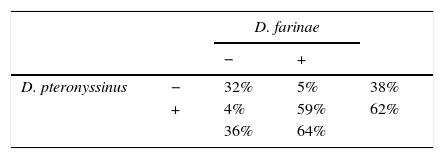
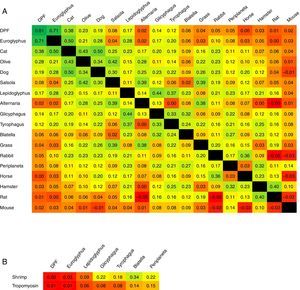
The concordance of sensitisation between pairs of aeroallergens is shown in Fig. 2. Concordance between D. pteronyssinus and D. farinae was very good (κ=0.81, Table 4) and these allergens have been treated on a pooled basis (DPF) in this study. Concordance of sensitisation between DPF and Euroglyphus was good, but decreased markedly to poor with SM and with cockroaches. Concordance was somewhat better among SM (moderate–fair) and between SM and cockroaches (fair). Unexpectedly, concordance between the two species of cockroaches was only fair. Concordance between shrimp and shrimp tropomyosin was good (κ=0.62). Concordance of sensitisation between shrimp or tropomyosin and DPF was null, and mildly improved progressively (poor–fair), especially for shrimp, with SM and with cockroaches (Fig. 2B). Concordance among furry animals was moderate for cat–dog and fair to poor, or even null, for all other pairs of allergens. Concordance was moderate for the pairs Euroglyphus-cat, cat-olive and olive-salsola.
Magnitude of κ reported pair-by-pair between (A) ADHA and other important aeroallergens ordered by their frequency of sensitisation in our population (parallel sensitisation could be higher for prevalent allergens); and (B) shrimp or tropomyosin and arthropod related aeroallergens (mites and cockroaches). The colour scale in squares expresses the intensity of concordance (κ) from higher in dark green, to lower in bright red, with intermediate values in a continuous green-yellow-red scale. Intersection square between DPF-DPF shows κ between D. pteronyssinus and D. farinae.
We have confirmed that ADHA are the main aeroallergens in paediatric patients in our area. Significant sensitisation to SM has been observed. Cockroaches and furry animals other than cat or dog have less impact in our patients. Pollens and moulds play an important but secondary role. Allergic polysensitisation is the usual pattern in this paediatric sample.
Covariation of sensitisation is assumed to increase with parallel sensitisation (prevalent allergens in an area, with similar allergenicity and level of exposure), cross-reactivity (immunologically related allergens) or a mixture of both events.7 Accordingly, CS should be low if cross-reactivity is limited. We have assumed that CS equates to concordance of sensitisation to the involved allergens. To the best of our knowledge, this is the first time that κ has been used to explore CS in a population. Accordingly, the very good κ between D. pteronyssinus and D. farinae should reflect the compounded result of both high parallel sensitisation and high cross-reactivity; this is why we have decided to treat them on a pooled basis. The good concordance between DPF and Euroglyphus is probably related to their well-known cross-reactivity,10,11 given that Euroglyphus is far less frequently recovered from homes than DPF in our area.15–17 Sensitisation to SM was prevalent and, although it could be partially related to cross-sensitivity with DPF, the low κ observed in our study and the reported presence of SM in homes in our area15–17 point to the existence of some degree of real primary sensitisation. This finding agrees with the observations of other studies.2 Concordance between DPF and other invertebrates (cockroaches or shrimp) was about null, reflecting very low cross-reactivity. Concordance (and possibly cross-reactivity) was somewhat better among SM, cockroaches and shrimp. The lower than expected κ between the two species of cockroaches indicates that cross-reactivity is not evident.
Concordance was remarkable between shrimp and shrimp tropomyosin, showing cross-reactivity between the whole shrimp allergen and its major component. Concordance of shrimp tropomyosin with mites or cockroaches was very low, disclosing a very low frequency of cross-sensitisation and possibly contributing to the low CS observed between DPF and other invertebrates in this study.
Other pairs of allergens with relevant CS may be explained for different reasons: possibly some parallel sensitisation and cross reactivity for cat-dog or olive-salsola, or mainly some parallel sensitisation for cat-olive. Concordance between Euroglyphus-cat is puzzling, because it is higher than between DPF-cat.
Our study has several possible limitations. Firstly, we only included a selection of SM in our skin test panel. Other mites such as Acarus siro, Blomia tropicalis or Chortoglyphus arcuatus have not been tested in our patients because they are presumed to be irrelevant in our area.2,15–17 Secondly, we studied a convenience sample which we consider sufficiently representative of the rest of the subjects in our area, but the sample is certainly not representative of subjects living in other places. Thirdly, we have analysed the qualitative results (positive or negative) of skin tests but not serum levels of specific IgE. We believe that skin test results are valid enough for our purposes and much more convenient for our patients. However, from a theoretical point of view, our results could be interfered with by problems with the test: falsely low CS could be produced if extracts for skin tests lack sensitivity or, contrarily, falsely high CS could originate from a lack of specificity (for example, contamination in extracts from other allergens).18 We assume that the quality of currently used commercial extracts for skin tests precludes such risks. It is worth remembering that our work is not aimed nor designed to know if sensitising allergens are clinically relevant, or if high covariation of sensitisation is due to co-sensitisation or to cross-reactivity. The answer to these questions is not easy and has not been addressed in this work.
In conclusion, in our area, with a high sensitisation to HDM and other ADHA, CS is limited for most of the pairs of allergens, pointing to a lesser incidence of cross-reactivity than could be expected, especially between DPF and SM or other invertebrates. Clearly cross-reactive allergens (D. pteronyssinus–D. farinae, DPF-Euroglyphus, shrimp-tropomyosin) have a high κ, showing the usefulness of this coefficient for measuring CS and its sensitivity (but not specificity) in detecting cross-reactivity.
Ethical disclosuresConfidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Protection of human subjects and animals in researchThe authors declare that the procedures followed were in accordance with the regulations of the corresponding Clinical Research Ethics Committee and with the guidelines of the World Medical Association and the Declaration of Helsinki.
FundingThere are no financial relationships or funding sources relevant to this article.
Conflict of interestThere are no conflicts of interest.
We thank Víctor Iraola, María Ángeles López-Matas and Jesús Garde for their helpful comments.