INTRODUCTION
Malnutrition and infections are the first causes of mortality all over the world, especially in developing countries. The association between infection and malnutrition has been known since the Middle Age: victims of the Plague were recognized by their malnutrition. In 1968, Scrimshaw1 proved that malnutrition propitiates infection, by observing also a decrease in infant mortality as a result of measles and diarrhea after food supplementation in a Guatemalan village. Immune competence in malnutrition has been studied since then.
It has been shown during the research that central and peripheral lymphoid organs show hypotrophy and even atrophy during malnutrition, affecting thymus, lymph nodes, spleen and tonsils as well2,3. While serum IgM, IgG and IgA remain normal, the findings have shown a decrease of secretory IgA4. Antipolysaccharide antibodies showed reduction too. Thymus-dependent T lymphocytes5,6 and CD4 positive cells are reduced with a tendency to revert the CD4/CD8 relationship in malnutrition7. Phagocytosis and chemotaxis by mononuclear and polymorphonuclear neutrophils were decreased8-12. Complement fractions are decreased in Kwashiorkor malnutrition13 and normal in protein-calorie malnutrition without hepatic involvement14,15.
We had not found in our research of the literature studies about serum IgE levels in malnourished patients without associated parasitic infestations.
OBJECTIVES
Evaluation of serum IgE levels in children with primary moderate protein-calorie malnutrition compared to levels in well nourished children of the same age.
PATIENTS AND METHODS
Sixty-eight patients were submitted to evaluated to this study, however eighteen patients were selected, because the others had parasitic infestations. The patients were children between 2 and 4 years old. Standards for inclusion into the study are as follows: absence of associated parasitic infestation as proved by stool examination on three different occasions, no immunization or blood transfusion during the last three months; no current medication; weight deficit due to primary food intake deficiency between 25.1 and 40 %16,17; normal height18; absence of edema; no current infection observed by medical history, physical examination, white blood cell count and evolution over the last 15 days after the blood collection. As a control group, 15 children at the same age were chosen, the same standards for inclusion was applied except that they were well nourished18. Blood collection was performed only if the children needed some other blood examination during the course of their medical follow-up, as observed in their medical records. All children parents from both groups agreed that part of the blood collected could be used for research purposes, if necessary.
Serum IgE was analyzed in vitro from peripheral blood using the immunoenzymatic method. All determinations were made twice.
RESULTS
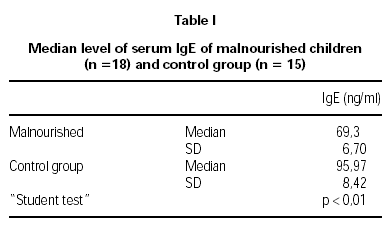
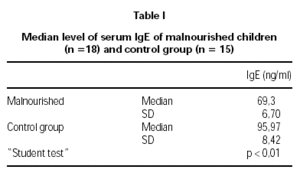
The serum IgE levels in malnourished children were 69.30 ± 6.70 ng/ml and 95.97 ± 8.42 ng/ml in the control group, showing a significant reduction (p < 0.01) in children with malnutrition. In both groups, stool examinations for parasitic infestation, collected on three different occasions, were negative (table I).
DISCUSSION
The results in the present study have showed a decrease in serum IgE levels in children with moderate malnutrition. We have studied patients with primary protein-calorie malnutrition, that is, as a result of insufficient food intake.
It is known that the humoral response is kept conserved in malnutrition, however there are not studies about IgE levels in malnourished patients without associated parasitic infestations, probably because the majority of these patients suffers from helminthiasis, as so we observed initially in this study. No children with parasitic infestations were included because helminthic infections are accompanied by an increase in serum IgE levels. IgE is important in the defense mechanism against parasites, either coating microorganisms which allow opsonization or increase intestinal peristalsis. Researchers have observed that helminthic infestations stimulate IL-4 and polyclonal IgE synthesis, the latter however an IgE of lower specificity, increasing these patients' susceptibility to helminthic disease8,9. These findings, even though in low socio-economic conditions and inadequate sewerage are without any doubt, responsible for the great incidence of parasitic disease in malnutrition. It is likely that this was the reason which made difficult the selection of malnourished children without parasitic infestation and associated infections for the present study.
No relation between the prevalence of asthma and socio-economic level has been found by various authors in the USA21-25; others found higher prevalence in well off families26 while some others found greater prevalence in poorer families27. In the United Kingdom, asthma is believed to be more frequent in children belonging to well off families28-32.
Prevalence of atopic diseases has increased over the past two decades, mainly in industrialized countries, including permanent sensibilization to air allergens35. Despite this, no reports of greater prevalence or increase in prevalence of atopic diseases have been released for malnourished individuals, even though malnutrition is most of the times due to marginalization of the population in regions with sudden increase in industrialization.
Harris JM et al36 observed that the association of environmental factors like house dust, lesser hygiene and larger families may, on one hand, increase the exposition to infections, while on the other, promote protection against atopic dermatitis. Those individuals with predominance of Th1 through immunologic necessity because of infections, are probably also lesser producers of citocinas synthetized by Th2 which induce IgE production.
During studies on the vascular permeability in rats which were either submitted to hypoproteic or normal diets, Cunha MG et al37 did not observe differences in mediator liberation during anaphylactic reactions. Though, they demonstrated that rats on deficient diets had significantly lower IgE levels, suggesting that the lower airway reactivity in these animals resulted from low levels of anaphylactic antibodies.
It is possible that adaptive mechanisms may occur in malnourished individuals in an attempt to save amino acids for anaphylactic antibodies which are apparently not as important as protein destined to synthetize cytoplasm and defense antibodies for infections.
It is concluded that children with moderate primary protein-calorie malnutrition have serum IgE levels, which are significantly lower than those in well-nourished children of similar age group. These findings are coherent with those in the literature, taking in account that there are less atopic reactions in malnourished patients.