INTRODUCTION
There is ample evidence that the prevalence of seasonal and perennial allergic rhinitis is increasing worldwide. Patients with these conditions are frequently treated with antihistamines and/or steroids which alleviate symptoms but do not provide any cure. Allergic symptoms may certainly be reduced by avoidance measures with some success; however, aeroallergens (particularly pollens) are the most difficult to avoid. The only allergy treatment available that is capable of providing long-term relief is specific immunotherapy (SIT)1 which is thought to act via stimulation of the Th1 lymphocyte response or by anergy of Th2 activity2. SIT may also prevent or slow down the "allergic march" in children by reducing the development of asthma3 which unfortunately is still a life-threatening disease.
Useful advances in the design of SIT vaccines have resulted in improved efficacy and tolerability. Conversion of allergens to allergoids (ie chemical modification of allergens) increases the potential tolerability of a vaccine by reducing the reactivity with specific IgE whilst maintaining the ability to induce specific IgG4. Depot adjuvants provide a slow allergoid release mechanism which both assists safety and promotes the immune response. Most SIT vaccines are now standardised, providing reproducible potencies and thus encouraging efficacy and tolerability.
This study utilises an L-tyrosine adjuvanted allergoid vaccine that is specifically designed for pollen allergy SIT (TA Mix top, Bencard Allergie GmbH/Allergy Therapeutics Ltd). The vaccine is fully standardised, meeting European requirements5,6. Treatment with this vaccine ensures that the pollen sensitisation profile of an allergic patient may be matched via the prescription of a patient-specific mixture. Allergoids included in this vaccine were derived from the following pollens: a 12-grass/cultivated rye mixture, birch, birch/alder/hazel mixture, mugwort and plantain. Treatment with this vaccine typically consists of a 3-dose initial course and a 3-dose maintenance course.
A further potential benefit of this vaccine is the use of the adjuvant L-tyrosine, a naturally occuring amino acid; L-tyrosine is fully metabolised giving a clear safety advantage over older adjuvants such as alum7,8. In the past, efficacy and tolerability of this short-term immunotherapy has been documented in several studies9,10. However, the small patient numbers used in these studies put some limitations on the clinical relevance of this data. We report the results of a multi-centre study where this short-term immunotherapy was applied to large patient numbers in a clinical practice setting.
PATIENTS AND METHOD
In this study 1808 patients with symptoms of allergic asthma and/or allergic rhinitis and/or allergic conjunctivitis were recruited from 200 centres in Germany. These conditions were diagnosed to be essentially due to sensitisation by combinations of pollens from the following flora: a 12-grasses mix and cultivated rye, birch/alder/ hazel trees, or the weeds plantain or mugwort.
Patients received subcutaneous injections of a pollen allergoid mixture according to their individual sensitisation profile, as prescribed by their physician. Pollen extracts were semi-purified, modified with glutaraldehyde and adsorbed onto 3 % L-tyrosine. A summary of those formulations that were prescribed is given in the results section. Initial therapy was given using three injections at 1-2 week intervals, consisting of 0.5 ml allergoid/L-tyrosine suspension at 300, 800 and 2000 standardised units (SU) respectively. This was followed by maintenance therapy with three further top doses (2000 SU) given at injection intervals of 1-4 weeks. All initial treatments and the majority of maintenance treatments (82 %) were administered before the start of the appropriate pollen season.
Efficacy was recorded in two ways. Firstly, an opinion of therapeutic success given by the physician was taken using a five-point scale ("very good" to "worsened"). Secondly, the consumption of anti-allergic medication was monitored, comparing to that used in the previous pollen season. The pollen seasons were not significantly different regarding severity.
Physicians assessed the tolerability of both initial and maintenance courses by employing a three-point scale ("very good", "good", "less good"). Local and systemic reactions were recorded for both courses.
Statistical evaluation for differences in continuous data was done by the Wilcoxon signed rank test and differences in frequencies or percentages were calculated by the chi2- test.
RESULTS
A total of 1808 patients were studied including 55 % females and 45 % males. The mean and median ages were 30 years (standard deviation = 14 years), with the majority of patients between 10 and 39 years of age. The youngest subject was 6 years and the oldest 67 years old. A minority of patients (31 %) had undergone previous hyposensitisation treatment, the vaccines most frequently formulated with grasses/rye, birch or alder pollen extracts (50 %, 34 % and 20 % of patients, respectively).
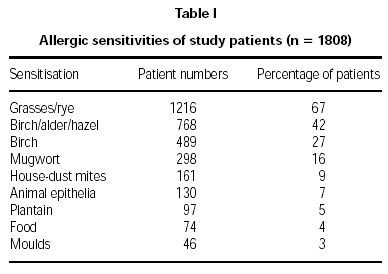
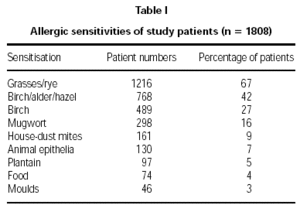
Diagnosis of pollen allergy was made using a combination of tests. These comprised: skin prick tests 89 %, case history 83 %, RAST 40 %, and provocation in 11 % of patients. Allergic sensitisations of the patients are displayed in table I.
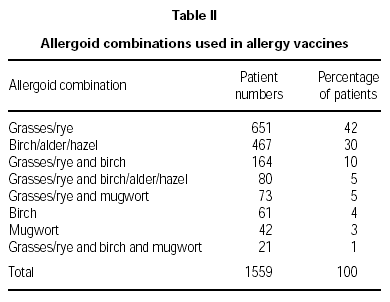
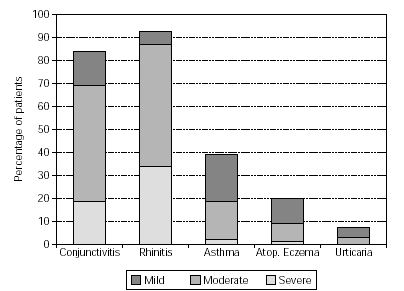
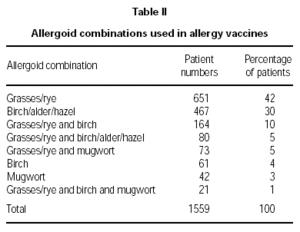
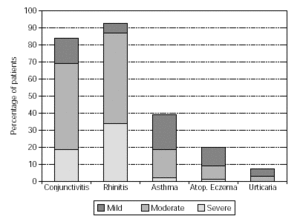
Physicians prescribed vaccines containing appropriate combinations of allergoids to match the individual sensitisations of the patients. The majority of patients (72 %) was treated with grasses/rye (n = 651) or birch/alder/hazel (n = 467), respectively. For brevity, only those combinations prescribed for 20 or more patients are illustrated (table II). A summary of the symptoms presented by the patients in the pre-treatment pollen season is given in figure 1. Conjunctivitis and rhinitis predominated, followed by asthma occuring in 39 % of the patients and atopic eczema and urticaria in smaller minorities (18 % and 6 % respectively).
Figure 1.--Symptoms of patients in pollen season prior to treatment.
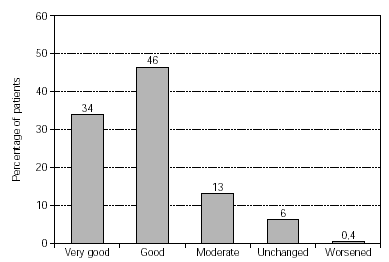
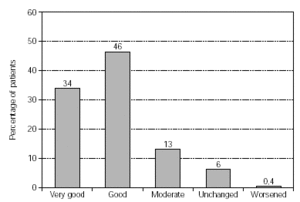
Efficacy of treatment was reported from 1558 patients (fig. 2). Therapy success was found to be either very good or good in 80 % of the patients, and a moderate success was reported in 13 %.
Figure 2.--Success of therapy (physician's evaluation; n = 1558).
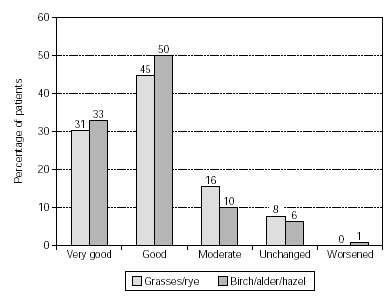
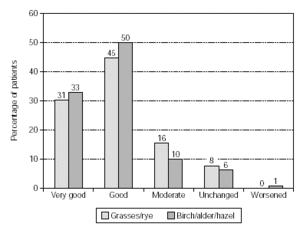
In patients treated with grasses/rye (n = 578) or birch/alder/hazel (n = 392) similar results were reported with very good/good therapy success in 76 % and 83 % of patients. Moderate success was achieved in 16 % and 10 %, respectively (fig. 3).
Figure 3.--Success of therapy in patients treated with grasses/rye (n = 578) or birch/alder/hazel (n = 392).
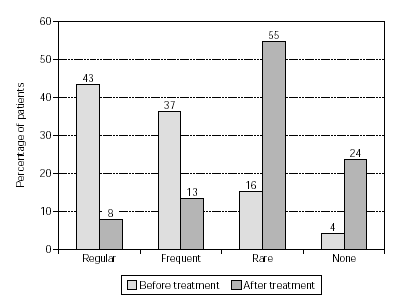
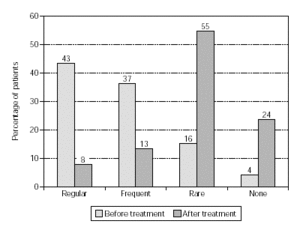
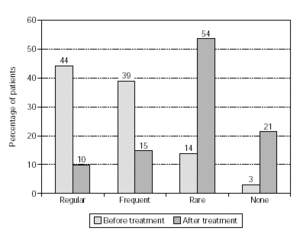
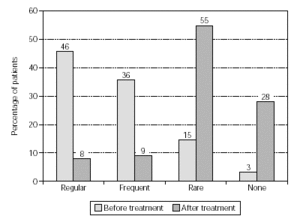
Efficacy, as quantified by reduction in the use of anti-allergic medication, is shown in figure 4 (n = 1554). Medication consumption sharply diminished: regular use went down from 43 % to 8 % of the patients, and frequent use dropped from 37 % to 13 %. The overall reduction in medication was a highly significant effect (p < 0.001) which was also observed in patients treated with grasses/rye or birch/alder/hazel (p < 0.001; figs. 5 and 6).
Figure 4.--Use of anti-allergic medication in pollen seasons before and after treatment (n = 1554).
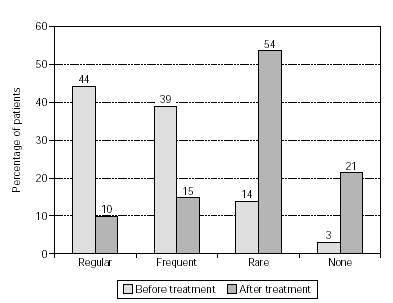
Figure 5.--Use of anti-allergic medication in pollen seasons before and after treatment from patients treated with grasses/rye (n = 393).
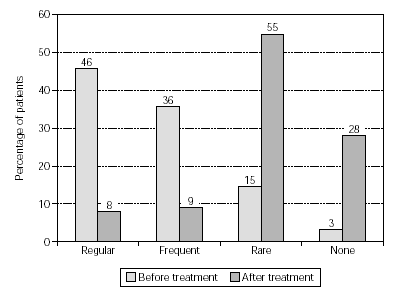
Figure 6.--Use of anti-allergic medication in pollen seasons before and after treatment from patients treated with birch/alder/hazel (n = 575).
These reductions were also seen in a further analysis dividing patients into an "early" and "late" therapy group, depending on the date of their final maintenance course injection. The first group (n = 821, 47 %) had the last injection before the end of February and the second group (n = 927, 53 %) from March onwards. In both groups medication consumption was reduced (p = 0.46). Therapy success was estimated "very good" in 35 % and 33 % of patients, and a "good" rating was achieved in 44 % and 48 % ("early" and "late" groups respectively). A moderate success was reported by 13 % ("early" group) and 14 % ("late" group). Unchanged patients were seen in 7 % ("early" group) and 5 % ("late" group). Worsening of symptoms was reported in 1 % ("early" group). The therapy was estimated to be equally successful in both groups (p = 0.36).
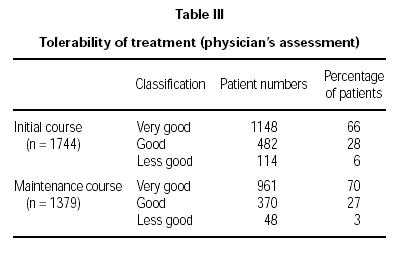
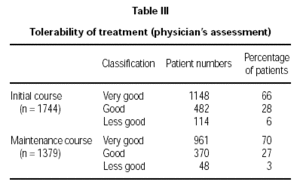
Tolerability was reported by the physicians in three classifications (table III). Data from 1744 patients (initial course) and 1379 patients (maintenance course) were evaluated.
The tolerability was clearly high with a total of 94 % of the patients with a "very good" and "good" rating treated with the initial course, and a 97 % total for the same ratings for the patients at the maintenance stage. This slight difference was statistically significant (p = 0.04).
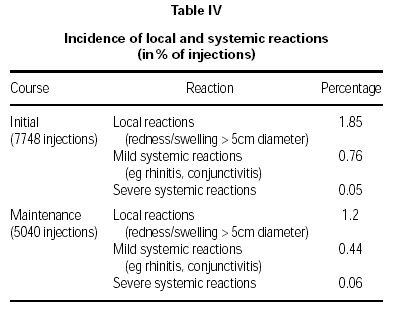
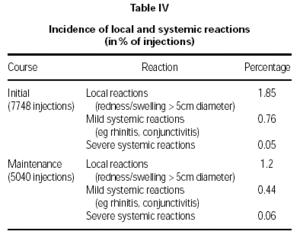
Local and systemic reactions following treatment with both the initial and maintenance courses are summarised in table IV. A total of 12,788 injections was evaluated (7748 initial course, 5040 maintenance course).
The vast majority of reactions were of a mild nature, and most events either did not require medication or were just treated by cooling. Patients treated with the maintenance course showed a significant reduction in the incidence of local reactions compared to that found with the initial course (p = 0.02). There were no significant differences in local or systemic reactions comparing the pre-seasonal and co-seasonal therapy group.
Severe systemic reactions were reported from 6 patients corresponding to a percentage of 0.05 % (initial course) and 0.06 % (maintenance course). In 3 cases the reaction was found to be local. The remaining 3 systemic reactions occurred during initial therapy; two reactions were correlated to the injection, 1 reaction was probably correlated. From these 3 reactions classified as "severe" one anaphylactic reaction occurred and was successfully treated (Tavegil, Solu-Decortin i.v.).The symptoms disappeared after 30 minutes. The second of these "severe" reactions was sublingual itching whereas a description of symptoms was missing in the third case. No serious systemic reaction was reported.
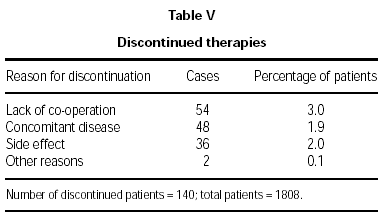
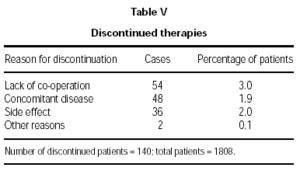
Therapy was discontinued for 140 patients (7.7 %), the major reasons being lack of co-operation or a concomitant disease, as shown in table V.
DISCUSSION
The short term immunotherapy in this study has some special features regarding its potential use. Firstly, there is the capability of patient specific mixtures to suit virtually all patients with allergy to common pollens, ie those monosensitised and polysensitised. Secondly, the allergoid/L-tyrosine combination offers efficacious and safe treatment with a comparatively low number of injections, which assists compliance and saves time for both physician and patient.
Impressive efficacy was judged by physicians, with a positive overall response for SIT given for 93 % of the patients, leaving only 6 % with symptoms unchanged and 0.4 % worsened. The results were confirmed in a subgroup analysis. Patients treated with grasses/rye or birch/alder/hazel achieved very good/good therapy success in 76 % and 83 % respectively. Efficacy was also demonstrated by substantially reduced medication consumption, particularly regarding regular and frequent use. The effect was statistically highly significant (p < 0.001). This reduction in antiallergic medication was also reported in patients treated with grasses/rye or birch/alder/hazel. The success of therapy and reduced consumption of antiallergic medication was equally documented in an "early" and "late" therapy group with respect to the last injection before pollen season.
Similar results, although with small patient numbers were reported elsewhere9,10. The authors reported a very good/good therapy success in 73 % (grasses/rye) and more than 80 % (birch/alder/hazel) respectively. Moreover a marked reduction in medication consumption was reported in these studies with grass- or tree-pollen allergic patients9,10.
Tolerability of the treatment was encouragingly high; "very good/good" ratings were given by 94 % of the patients after the initial course, and 97 % of the patients after the maintenance course. The difference was statistically significant (p = 0.04).
Local and systemic reactions were mainly mild. Local reactions from the maintenance course were significantly lower than those from the initial course (p = 0.02), which is possibly an indication of some desensitisation already resulting from the first three injections. Severe systemic reactions were very limited (initial course 0.05 %; maintenance course 0.06 % of injections), with no statistical difference between therapies. No serious systemic reactions were reported.
Some maintenance courses were administered co-seasonally. One may speculate that the additional exposure during pollen season might be detrimental to safety. But those patients given co-seasonal maintenance therapy showed no significant differences in local or systemic reactions in comparison to the pre-seasonal therapy group. It may be concluded that the good tolerability of this short-term immunotherapy which has been reported elsewhere9,10 was confirmed in this study.
In summary, this study has shown that short term SIT has provided a successful treatment of pollen-allergic patients with allergic asthma and/or allergic rhinitis and/or allergic conjunctivitis. Since this data involved large patient numbers the results of smaller studies could be confirmed.
In a clinical practice setting, a pollen allergoid/L-tyrosine vaccine has provided high efficacy with a low incidence of adverse events which were mainly mild in nature. The convenient dosage with 6 injections could improve compliance and may support wider application of specific immunotherapy (SIT) in future.