RESUMEN
El componente inflamatorio crónico del asma ha justificado el manejo con antiinflamatorios de tipo esteroide inhalados solos o en combinación con β2 de acción prolongada para manejo habitual del asma moderada crónica persistente (AMCP). El objetivo fue comparar los efectos de beclometasona frente a salmeterol con beclometasona en IDM sobre el potasio sérico, el intervalo QTc y en los valores de las enzimas del músculo cardíaco CPK-MB en niños asmáticos sin crisis del servicio de alergia del Hospital Infantil de México Federico Gómez. Se hizo un ensayo clínico prospectivo, longitudinal, ciego, cruzado, comparativo de dos tratamientos. administrados de forma aleatoria en diferentes tiempos en un mismo grupo de 30 pacientes de 7 a 17 años con AMCP de acuerdo a la clasificación del GINA. A los pacientes seleccionados se les determinó potasio, CPK-MB y trazo de ECG antes y después de las 6 semanas de tratamiento (salmeterol 100 μg/día con beclometasona 400 μg/día (Sal-Beclo) y beclometasona (Beclo) sola a la misma dosis. El inicio del tratamiento fue de tipo aleatorio quedando 14 pacientes con Sal-Beclo y 16 con Beclo, con 1 semana de lavado después del primer tratamiento para continuar el grupo que inició con Sal-Beclo con Beclo y el de Beclo con Sal-Beclo.
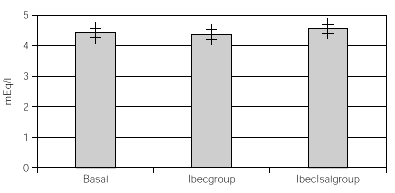
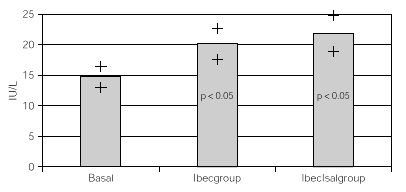
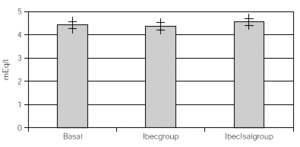
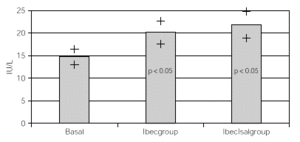
Resultados: hubo 9 niñas y 20 hombres con una media de 11 ± 2,18 años. Con K basal de 4,57 ± 0,43 mEq/l con Beclo de 4,38 ± 0,39 y con Sal-Beclo de 4,38 ± 0,40. La CPK-MB basal fue de 14,75 ± 8,45 después con Beclo 20,10 ± 6,9 y con Sal-Beclo 21 ± 8,05. Los cambios en la CPK-MB basal frente a CPK-MB con Beclo y la CPK-MB basal con Sal-Beclo se obtuvieron valores significativos (p < 0,05) El QTc basal fue de 0,416 ± 0,02 mseg después de Beclo 0,425 ± 0,027 y con Sal-Beclo de 0,415 ± 0,029 (p > 0,05).
Conclusión: la administración de 400 mg al día de beclometasona sola o en combinación con 100 mg/día de salmeterol en inhalador de dosis medida por 6 semanas en el tratamiento habitual de niños con ACMP no induce cambios cardiovasculares importantes a pesar de haberse visto una elevación significativa de la CPK-MB en niños sin crisis.
Palabras clave: Asma infantil. Beclometasona. Beclometasona-salmeterol. Potasio sérico. CPK-MB, ECG. Cardiotoxicidad.
SUMMARY
Asthma morbidity and mortality has increased. One of the possible causes is the excessive use of beta agonists. The aim of this study is to compare the effects of six week treatment with beclomethasone alone (Ibec) or the combination of beclomethasone-salmeterol (Ibe + Isal) on serum potassium (K), CPK-MB and ECG in children suffering asthma. It was a prospective, randomised, open cross-over trial. Patients received either Ib2 (2 puff/12 hr, 100 μg per puff) or Ibe + Isal (B 2 puff/12 hr, 100 μg per puff and S 2 puff/12 hr, 25 μg per puff) with dose meter inhaler by 6 weeks, with a four-week wash-out period between the treatments. K, CPK-MB and ECG were assessed at baseline, and after each treatment period. There were 9 girls and 20 boys, aged 11 ± 2.18 (mean ± SD) years, baseline K was 4.57 ± 0.43 mEq/l, after B K 4.38 ± 0.39 IU and after BS K 4.38 ± 0.40. The CPK-MB level were baseline 14.75 ± 4.5, after B 20.10 ± 6.9 and after BS 21 ± 8.05 (p < 0.05). Baseline QTc was 0.416 ± 0.02 msec, after B 0.425 ± 0.027, and after BS 0.415 ± 0.029. We conclude that the treatment of children with asthma with 400 mg per day of Ibec or concomitantly with 100 μg of Isal for 6 weeks does not alter the serum K+ or the QTc. However, the CPK-MB has a significant increment with both treatments but without clinical and/or ECG changes. We can't affirm that Ibec or Ibec plus Isal have a cardiotoxic side-effect by the only presence of high levels of CPK-MB. We agree that it is necessary a close follow up of these apparently asymptomatic patients not induce important cardiovascular changes although CPK-MB was increased.
Key words: Asthma in children. Beclomethasone. Beclomethasone-salmeterol. Serum potassium levels. CPK-MB. ECG. Cardiotoxic side-effect.
INTRODUCTION
Asthma is the most frequent chronic lung disease on childhood (1). Bronchospasm, inflammation and bronchial hyperreactivity characterize it. According to the Global Initiative for Asthma (GINA) it is considered a Chronic Inflammatory disorder of the airways with recurring episodes of coughing, wheezing, chest tightness and difficult breathing with airflow limitation on Forced Expiratory Volume in the first second (FEV1) as measured by spirometry and/or on Peak Expiratory Flow (PEF) (more than 15 percent) as measured by peak expiratory flow meter.
Asthma incidence and prevalence is not well known all over the world, however it is estimated that affects between 10 and 12 percent of children.
According to the frequency of daily and nightly symptoms, limitation of physical activity and pulmonary function test, chronic asthma has been classified on:
1. Mild intermittent (MICA).
2. Mild persistent (MPCA).
3. Moderate persistent (MCPA).
4. Severe persistent (SPCA).
1. MICA is characterized by less than 1 time daily symptom per week, less than 2 nighttime symptoms per month and with a PEF ≥ 80 % from predicted, with < 20 % variability.
2. MPCA is characterized by ≥ 1 daily symptoms per week but < 1 time a day, > 2 nighttime symptoms per month and with a PEF ≥ 80 % from predicted with 20-30 % variability.
3. MCPA is characterized by daily symptoms and more than 2 nighttime symptoms per week and with a PEF > 60 %- < 80 % from predicted, with > 30 % variability.
4. SPCA is characterized by frequent daily and nighttime symptoms with exacerbations with a PEF ≤ 60 % from predicted, with > 30 % variability.
The aim of this classification is to instruct for a step-to-step treatment over a correct diagnosis, with symptoms control and avoiding future inflammatory problems that can permanently limit or affect the lung function (2, 3). Due to its evolutive chronic inflammatory pattern, it can cause irreversible structural changes in the airway (remodeling) that in the future will lead to the chronic obstructive pulmonary diseases of the adult patient (4).
The actual treatment is established with quick-relief drugs, controllers or preventives. The first ones are called rescue drugs, in this group are included the short acting β2 agonist like salbutamol or terbutaline. These ones had proved an undoubted efficacy specially when administrated by inhaled way on the exacerbation because of its fast bronchoconstriction action (3, 5, 6). The difference between the quick-relief drugs and the controllers is that the last ones minimizes the bronchial hyperreactivity by its stabilizing action over the cell's membranes, and this avoids the cellular degranulation and thus preventing the release of vasoactive and inflammatory mediators when in touch with several triggering stimulus like allergens, viral infections, pollution, etc. In this controllers group are the cromones, inhaled steroids and anti-leukotrienes (2, 6, 7).
According to the GINA asthma management and prevention guidelines, it is recommended to use preventive drugs for long time periods, reviewing every 3 months the asthma intensity to adjust the treatment. Non steroid anti-inflammatory drugs like cromones, or nedocromil and anti-leukotrienes are indicated on MPCA and, in MICA preventive drugs are recommended. In case of non-response, steroid drugs are indicated. According to the severity score of symptoms, the doses vary from 400 μg per day to 1,000 μg per day on SPCA of Inhaled beclomethasone (Ibec) (2, 3, 7, 8). With the new arrival of long-acting β agonist (formoterol and salmeterol), symptoms have improved without increasing steroid doses (7, 9). Salmeterol is a saligenin that has a salbutamol-like structure with a few differences on its molecular structure because it has an aliphatic long lateral chain and a wide terminal catechol that binds repeatedly to the active site of the adrenergic receptor. Salmeterol is safe and effective on children between 4 and 11 yr. at a daily total dose of 100 μg Meter Dose Inhaler (MDI) (10-14) for 12 weeks with a Meter Dose Inhaler.
Unfortunately the inhaled β2 adrenergic drugs (nebulized or MDI) have some side-effects like cardiac overstimulation, muscle shiver and mild hypokalemia. Its mechanism of action is by stimulation β2 receptors, causing smooth bronchial muscle relaxation and cardiovascular stimulation. When parenterally administered they have more side-effects over serum K+ because they act over the Na+/K+ Pump (1, 6).
Long term treatment with long-acting b2 agonist improves the symptoms and lung function, when its associated with an Inhaled Steroid (IS) MDI (7, 12, 15).
The steroid's mechanism of action is by interfering on the arachidonic acid metabolism, inhibiting phospholipase A2 activity and therefore interrupting the synthesis of bronchoconstrictor mediators like leukotrienes, thromboxanes and PAF that produces bronchial mucus and edema (17).
With the arrival of IS, the side effects had been decreased like suppression of the hypothalamic-hypophysial-adrenal-axis (HHAA) and its effect over electrolytes and water, bone metabolism and children's height. Beclomethasone is an IS and its most common side-effect is oropharynx candidiasis and/or dysphony, that are present in up to 29 % of the people using it and reducing these effects with the use of space chambers (16, 17). These space chambers are extension tubes between the MDI device and the patients' mouth, which prevents the direct hit on the patients' oropharynx of the drug and driving it to the central airways. The therapeutic index or drug safety over the HHAA is described with 400 μg per day of beclomethasone in children over 6 yr. for six month periods or longer (10, 16, 17). If the short-acting β2 agonist have side-effects over the K+ (18, 19), CPK-MB (18-24) and ECG (14, 20, 25-28), the use of a β2 agonist like salmeterol that binds repeatedly to its receptor by its long lateral chain when associated with an IS can increase the IS side effects (12, 14). Therefore with the large number of drugs available for the treatment of MCPA on children over 5 yr., we are worried that just a few studies had been developed for the correct understanding of the side-effects over K+ and cardiovascular system when an IS and a long-acting β2 agonist are concomitantly used.
MATERIAL AND METHODS
A clinical, parallel, randomized, prospective, longitudinal, single-blind, crossover trial comparing inhaled beclomethasone (Ibec) versus Ibec plus inhaled salmeterol (Isal) for 6 weeks on children between 7 and 17 yr. with MCPA was developed at the Hospital Infantil de México "Federico Gómez", Allergy Department.
All patients had MCPA diagnosis according to GINA criteria and were not pretreated with any drug that altered the serum K+ levels like diuretics or steroids or the cardiac conduction system like antihistamines. They don't have cardiovascular diseases nor airway infection or any other chronic lung pathology. Patients that have a history of any clinical significant adverse experience or sensitive to Ibec, Isal or their components were excluded. Patients that can't use correctly a space chamber or an MDI device, or have an altered basal serum level K+ (over 10 % of normal range) or CPK-MB (over 25 %) or ECG trace or do not want to sign an informed consent were excluded too.
Once the ethics committee approved the trial and after the parent/guardian signed the informed consent, the patients were trained in the use of MDI and space chambers. The technique and treatment adherence was evaluated each visit by an interview and measuring the quantity of water displaced by the MDI canister before and after the visit. The initial treatments were randomizedly assigned with 16 patients on group A using Ibec plus Isal and 14 on group B using only Ibec, for 6 weeks. After the treatment, both groups had a 1 week washout period and started the crossover treatment with group A using only Ibec and group B using Ibec plus Isal, for another 6 week period.
Serum K+ levels were determined by an electrode method with a flamephotometer 343 (made in USA) with a 1 ml blood sample. Normal ranges were considered between 4.5 to 5.5 mEq/l.
CPK-MB was determined with a Monarch 2000 (made in USA) equipment with a 2 ml blood sample. Normal ranges were considered between 0 to 15 IU/l.
The ECG trace was performed with a Hewlett Packard 474 A Writer II electrocardiograph (made in USA). All electrocardiographic leads as well as ST, QRS, and QTc segments and intervals were obtained. A pediatric cardiologist evaluated ECG's.
Elimination criteria were when patient, had any adverse experience related to the drug K+ and/or CPK-MB levels over 25 % and/or QTc out of normal range. Patients were followed up for 1 week or until complete recuperation.
Sample size was determined according to Del Rio and Sienra-Monge data (19) where basal serum K+ was 4.7 ± 0.52 and the final one was 3.7 ± 0.49 mEq/l. The sample size was 30 patients for each group of treatment with and alpha error of 0.05 and a power of 90 % with the program Primer of Biostatics version 4.0 based on Glantz's book (30).
For the statistical analysis, measures of central tendency with dispersion and univariate variance analysis were used and considering type and order like factors of treatment and Post Hoc of Turkey and Bonferroni test in a SPSS version 8 program.
RESULTS
Considering that all patients had both treatments after the crossover, all data were overall analized as one set of 30 children within 2 treatment groups. One with inhaled beclomethasone (Ibecgroup) an another one with inhaled beclomethasone and inhaled salmeterol (IbecIsalgroup). From the 30 children, one was eliminated for address change. The remaining were 9 girls and 20 boys with a mean age of 11 ± 2.18 yr. When comparing basal and final K+, we found non significant differences on both groups. Ibecgroup basal 4.43 ± 0.43 and final 4.38 ± 0.39 (CI 95 % 4.24-4.5l); IbecIsalgroup basal 4.43 ± 0.43 and final 4.57 ± 0.40 (CI 95 % 4.43-4.70). Basal CPK-MB was 14.76 ± 4.52 IU/l, after treatment the final CPK-MB on the Ibecgroup was 20.10 ± 6.99 IU/l (CI 95 % 17.55-22.64) and the final CPK-MB on the IbecIsalgroup was 21.79 ± 8.05 IU/l (CI 95 % 18.85-24.73). Regarding on the ECG traces, the basal QTc was 0.4162 ± 0.020 msec on both groups, and after the treatment the Ibecgroup had 0.4252 ± 0.026 and the IbecIsalgroup had 0.4155 ± 0.028. The basal P-R was 0.293 ± 0.019 and after the 6 weeks was 0.293 ± 0.019 on the Ibecgroup and 0.130 ± 0.016 (p < 0.05).
Table I shows the medias, standard deviations and standard errors of serum K+, CPK-MB, QTc, P-R and Heart Rate. Figures 1 and 2 show the K+ and CPK-MB values with CI 95 %.
Figure 1.--Basal and post treatment levels of K+. Values are expressed as medias and CI (95 %)+.
Figure 2.--Basal and post treatment levels of CPK-MB. Values are expressed as medias and CI (95 %)+.
DISCUSSION
Szakacs and Mehlman (31) described the first deleterious effects of β agonists at the end of the 1960's using isoproterenol i.v. on adult patients on crisis. In 1961 to 1966 at United Kingdom, there was an association between nebulized isoproterenol on crisis and teenage deaths (33). On the following years, many authors (25-27) showed the isoproterenol's cardiotoxicity with an unspecific myocarditis with a final outcome of myocardial necrosis and an inflammatory infiltrate.
In the 1970's at New Zealand and despite the decrease of isoproterenol use, β2 agonist appeared as mortality cause when fenoterol was used and Supraventricular arrhythmias were noted with salbutamol, a short-acting β2 agonist (22).
Between the possible explanations of death among asthmatics because of the use of β2 agonist was the subestimation of the obstruction and to the excess of confidence with the use of these drugs that had as final outcome the obstruction worsening as a result of persistent inflammatory response and subsensitivity of β adrenergic receptors (34). From a metabolic point of view the hypoxemia, respiratory acidosis and the over use of β adrenergic drugs, directly cause hypokalemia (by the action of β2 agonist over the Na+/K+ pump) with disturbances of the cardiac rhythm by stimulation of the β2 adrenergic receptors and by reflex activation of the adrenergic mechanisms originating vasodilatation shown as tachycardia, cardiac arrhythmia or QTc prolongation (18, 23, 27, 28, 33).
In our experience, on a previous study, we showed a significant decrease without clinical relevance of the serum K+ and without QTc changes on 20 asthmatic children in crisis treated with nebulized salbutamol. Other authors like Papo (18), Katzs (32) and Shrestha (32) proved with ECG traces a safety level of continuous nebulized salbutamol of 0.150 μg/kg for 6 hr and correlating hypokalemia with doses over 2,400 μg.
Unfortunately we don't have enough studies evaluating the side-effects over the cardiovascular system with the use of long-acting β2 agonists as done with the short-acting β2 agonist, maybe because its recent marketing introduction. The cardiovascular side-effects reported with the use of salmeterol have been studied only on just a few patients (8 healthy and 8 asthmatic adult patients) using 100 to 200 μg of salmeterol, concluding the cardiovascular safety with Heart Rate and Blood Pressure as variables (29). If we can extrapolate the results of continuous nebulized salbutamol to salmeterol that has a similar mechanism of action but with a longer half-life, we found that our results over the serum K+ and ECG (QTc) are similar to the previous reported by Bremner (34) and Papo (18).
We can't fully compare our CPK-MB results with the previously reported of this cardiac muscle enzyme because they measured it on patients suffering a crisis and treated with nebulized salbutamol and we used stable asthma. However, as well as Maguire and Geha (21) found a CPK-MB increment on 9 of 15 children on crisis treated with continuous nebulized salbutamol and Craig (23) found the same results on the same conditions on 1 of 3 children, we cant state that this increment is a predictive factor for severe cardiotoxicity. Therefore we require more studies to determine the clinical significance of the CPK-MB elevations.
Considering that steroids have a catabolic action over the muscle tissue and can secondary increase the CPK, this can't explain the CPK-MB increment because a low dose of Ibec was used (400 mg). Unfortunately we didn't measure the other CPK fractions (MM, BB) and thus determine the catabolic effect over the muscle fibers.
High levels of CPK-MB suggest an acute myocardial lesion but these levels have to be correlated with the clinical symptoms and ECG changes to be relevant. So we have to do a close follow up of the patients with MCPA apparently asymptomatic under treatment with IS and/or inhaled salmeterol to determine its clinical significance.
We conclude that the treatment of children with MCPA with 400 μg per day of Ibec or concomitantly with 100 μg of Isal for 6 weeks does not alter the serum K+ or the QTc. However, the CPK-MB have a significant increment with both treatments but without clinical and/or ECG changes. We can't affirm that Ibec or Ibec plus Isal have a cardiotoxic side-effect by the only presence of high levels of CPK-MB. So, as Maguire, Katzs and Craig said, we agree that it is necessary a close follow up of these apparently asymptomatic patients to determine if at long term exist any condition that favor myocardial damage when using IS and/or long-acting β2 agonist when suffering asthma worsening or when needing systemic steroids for 5 days.