Severe delayed drug-induced skin reactions in children are not common but potentially serious. This article describes aspects concerning the etiology, pathogenesis and clinical manifestations of these processes; it presents three paediatric cases, namely STS (Steven Johnson Syndrome), TEN (toxic epidermal necrolysis), probably related to amoxicillin/clavulanate and ibuprofen and DRESS (a drug reaction with eosinophilia and systemic symptoms) secondary to phenytoin; and in relation to them, the diagnosis and the treatment of these processes are discussed and reviewed. The AGEP (acute generalised exanthematous pustulosis) is also reviewed. The aetiological diagnosis of severe non-immediate reactions is difficult, and the value of current allergological testing is not well defined in these cases. Diagnosis is based on clinical history, the empirical risk of drugs to trigger SJS/TEN or DRESS, and the in vivo and in vitro testing of the suspect drug. Skin biopsy confirms that the clinical diagnosis and delayed hypersensitivity tests, especially the patch test and the lymphoblastic transformation test (LTT), may be important to confirm the aetiological diagnosis, in our cases emphasising the latter. These diseases can be life threatening (especially DRESS and TEN) and/or have a high rate of major complications or sequelae (SJS/TEN). The three cases described progressed well without sequelae. All were treated with corticosteroids, which is the most currently accepted treatment although the effect has not been clearly demonstrated.
Severe delayed drug-induced skin reactions in children are less common than in adults, but potentially serious, with an estimated mortality risk of 10% for DRESS syndrome, 1–5% for SJS (Stevens Johnson syndrome), 25–30% for TEN,1 and less than 5% for (AGEP),2 and a high rate of major complications or sequelae, such as in SJS/TEN.1
This article first describes aspects concerning the aetiology, pathogenesis and clinical manifestations of these processes. Then, it presents three paediatric cases, namely STS, TEN and DRESS syndromes. Finally, in relation to them, the diagnosis and treatment of these proceses are discussed and reviewed.
DRESS SYNDROME is a rare, potentially life-threatening, adverse drug reaction with cutaneous manifestations and internal organ involvement, which occurs in both adults and children.3 The term DRESS was proposed by Bocquet et al.4
DRESS aetiology is generally regarded as a severe hypersensitivity to drugs and their reactive metabolites, which can be associated with enzymatic defects in drug metabolism.5 Its exact mechanism of production remains unknown.6 A type IVb7 hypersensitivity reaction has been proposed, but viral infections, particularly herpesvirus 6, have been shown to play a role.8 Reactivation of herpesvirus infection concurrent with drug hypersensitivity is considered specific of DRESS.9,10 It is also well established that individuals with specific HLA haplotypes are predisposed to develop this syndrome when exposed to certain medications.11 The incidence of DRESS is unknown but it has been estimated that the average population risk is between 1 in 1000 and 1 in 10,000 exposures to drugs.4,12
In the RegiSCAR (a multinational registration of severe cutaneous drug reactions), it was found that antiepileptic drugs, especially carbamazepine and lamotrigine, phenobarbital, phenytoin, valproic acid and zonisamide, were involved in 35% of cases; allopurinol in 18%; sulfonamides and dapsone in 12%, and other antibiotics in 11%, and that the average interval after taking the drug was 22 days, with differences between drugs.13 Adverse drug reactions usually begin within 2 months after intake of the offender drug, most often between 2 and 6 weeks after its first use4,14; however, symptoms may occur more quickly and be more severe in subsequent exposures.4
DRESS usually begins with non-specific prodromal symptoms such as fatigue, pruritus, and fever. An unexplained fever between 38 and 40°C may coincide with, or be preceded by several days by, the rash, which usually appears as a non-specific disseminated morbilliform maculopapular rash.6 The rash is present in 95% of patients,13 starting on the face and upper trunk, spreading down and then quickly developing a bluish tint. The eruption suggests a DRESS when affecting more than 50% of the body surface and/or it includes two or more of the following: facial oedema, infiltrated, scaling and purple lesions.15 Facial oedema occurs in about half of cases13; it is symmetrical, persistent and is accompanied by erythema. Almost half of the patients displayed swelling and pain of the mucous membranes, affecting usually just one mucosa (most often the mouth and pharynx), without progressing to erosion.
Eosinophilia (>700/mm) occurs in between 50 and 90% of cases, atypical lymphocytes, lymphoblasts or mononucleosis-like cells in between 30 and 70% of cases, increased alanine aminotransferase in up to 80% of cases and HH-V infection in between 40 and 60% of cases.15
Systemic signs include enlarged lymph nodes (30–60% of cases), haematological abnormalities with eosinophilia or atypical lymphocytes, and multiple organ dysfunction.12 The liver is the organ most frequently affected (60–80% of cases), but also occurring may be renal failure (10–30% of cases), impaired lung function, pneumonitis, heart failure secondary to myocarditis, neurological symptoms manifested as meningitis or encephalitis, gastroenteritis, and pancreatic or thyroid dysfunction.5,16
The rash and visceral involvement usually gradually resolve after drug withdrawal. The average recovery time is 6–9 weeks.16,17 In up to 20% of cases, the disease can persist for several months with a series of remissions and relapses.15
SJS was described in 192218 and TOXIC EPIDERMAL NECROLYSIS (TEN) in 1956.19 SJS and TEN are severe cutaneous adverse reactions affecting both children and adults.20 Considerable overlap exists in the clinical, aetiological and histopathological findings in both entities that justifies their being considered clinical presentations of a broad spectrum (or continuum) known as “SJS/TEN”.21 The main difference is in the percentage of body surface area (BSA) with peeling skin; in SJS epidermal detachment affects less than 10% of the BSA; in SJS/TEN overlap such detachment is between 10 and 30%; and TEN when affecting more than 30%.21 SJS, TEN AND SJS/TEN have been observed with an annual incidence of 2–7 cases per 1,000,000 population22–24; with SJS being more common than TEN, in a ratio of 3:125 and affecting all ages, including children, infants and neonates, the latter two groups comprising between 10 and 20% of reported cases.25 Mortality is lower in children than in adults,25 but sequelae are relatively common, being most commonly associated with mucosal involvement, especially ocular.26
The pathological mechanisms that induce skin damage in SJS/TEN are not fully understood, although it is considered as a delayed hypersensitivity reaction (type IV), reinforced by the fact that the reactions were resolved on drug withdrawal and occur more rapidly at reintroduction.27 The main pathological finding is widespread apoptosis of keratinocytes, but it is not known why certain drugs induce epidermal necrosis.20 In the initial phase cytotoxic T cells (CTL) play an important role.25 Abundant T CD8+ cytotoxic cells and natural killer cells (NKT-cell) have been seen in blisters, which could, through various cytotoxic proteins and cytokines such as soluble Fas ligand, perforin/granzyme, tissue necrosis factor (TNF)-alpha,25,28,29 and TNF-related apoptosis-inducing ligand, induce extensive apoptosis of keratinocytes in SJS/TEN. It is now accepted that among them, granulysin has a key role in the pathogenesis of SJS/TEN.28 The drugs can stimulate the immune system by binding directly to the major histocompatibility (MHC) I antigen and T cell receptor. This results in a clonal expansion of a population of drug-specific cytotoxic T cells which kills keratinocytes directly, or indirectly, through the recruitment of other cells that release lethal soluble mediators, including granulysin.30 Granulysin is a proteolytic enzyme produced and secreted by cytotoxic T lymphocytes, natural killer (NK) cells and NK/T cells. It has been identified as a molecule mainly expressed in patients with STS/TEN,31 correlating the levels of granulysin in blister fluid with the severity of disease. The mechanism by which cytotoxic T cells and NK cells are stimulated to release granulysin is unknown, although the results of one study suggest that the interaction between the receptor CD94/NKG2C of cytotoxic T lymphocytes and HLA-E, a MHC Ib class molecule expressed in keratinocytes in patients with SJS or TEN, can promote their degranulation and release.32
The hallmark of the STS/TEN is keratinocyte necrosis, ranging from partial epidermal necrosis to full-thickness necrosis. In early lesions, apoptotic keratinocytes are located in the basal layer of the epidermis, but in established lesions there may be overall thickness necrosis of the epidermis and subepidermal bullae. A lymphohistiocytic perivascular infiltrate containing a variable number of eosinophils is present in the superficial dermis.24
Many associations have been observed in recent years between STS/NET and human leucocyte antigen (HLA) class I and II alleles of MHC (major histocompatibility complex).11 Genetic susceptibility appears to play a crucial role in the development of SJS/TEN. HLA-B1502 has been associated with carbamazepine-induced SJS in a Chinese population,33 and HLA-B5801 with SJS/TEN induced by allopurinol in a Japanese population.34 A similar association has been identified for HLA-A*3101 allele, which is prevalent in a wide range of ethnic groups. In a cohort study in northern Europe, the risk of SJS/TEN induced by carbamazepine in the presence of this allele was 5–26% higher than in non-carriers,35 while in a Canadian study, in children with carbamazepine-induced hypersensitivity syndrome (HSS) the risk was 25 times higher in patients with presence of HLA-A*3101.36 The number of patients needed to study to prevent an adverse reaction was estimated to be 83.35 For these reasons, an expert panel has recommended that in view of the significant prevalence in many ethnic groups of HLA-A*3101 allele screening be performed for detection prior to treatment with carbamazepine.37
Moreover, drug reactions usually occur with great frequency in situations where there is an increase of chemically reactive metabolites.20 In this respect, we have identified polymorphisms in gene families of enzymes involved in detoxication, especially in the CYP450 family, which affects the kinetics of the drug and its toxicity.20 Polymorphisms in the CYP2C19 gene that encode an isoform of cytochrome P450 may increase the risk of SJS/TEN following the administration of phenobarbital, phenytoin, or carbamazepine.38 Moreover, some patients with STS/NET have a low N-acetylation capacity, which predisposes them to serious adverse skin reactions.39
Many cases of SJS/TEN are clearly linked to medications; however, approximately 20–25% of cases and probably an even higher proportion in paediatric patients cannot be clearly attributed to a drug.40,41
TEN is almost entirely attributable to drugs, but some cases of SJS/TEN can be caused by infection by Mycoplasma pneumoniae.42 Viral infections (coxsachie, influenza, Epstein–Barr, herpes virus 6 and 7, cytomegalovirus and parvovirus), bacterial (GABHS A) and rickettsia may be potential triggers or cofactors in association with drugs.25 It is believed that infections, including mycoplasma pneumoniae and cytomegalovirus, are the most common triggers of SJS/TEN, particularly in children.20,42–45
Over 200 drugs have been associated with SJS or TEN, most commonly sulfonamides, anticonvulsants, NSAIDs (oxicams), allopurinol and penicillin.43,46 Many studies in children describe aetiological factors in isolated case reports, although some series are reported.41,46–49 Many of these studies conclude that the drugs involved in STS/TEN are especially sulfonamides and anticonvulsants, including phenobarbital, lamotrigine and carbamazepine.47–50 Other drugs, such as penicillins, cephalosporins (rarely involved in adult SJS/TEN), valproic acid, NSAIDs (excluding oxicams in this age group), and acetaminophen, may have a potential risk in children.41,45–47,49 In contrast to adults, allopurinol, nevirapine and oxicam were not identified as causative agents in children.20 Prescribing patterns may be responsible for the changes in the causative drug20 in children versus adults.
SJS/TEN is an acute inflammatory disease characterised by fever, malaise, and skin and mucous membranes lesions, usually preceded by prodromal symptoms lasting between 1 and 14 days.25 The prodromal period is characterised by non-specific symptoms such as malaise, fever, ocular pruritus and dysphagia. Erythema, erosions, crusts and pseudomembranes develop gradually in the oral, ocular and genital mucosa. There is often painful inflammation of the mouth and genitals, usually a few days preceding the skin lesions.21,24,50,51 Skin injuries vary in severity, from red macules, papules which sometimes coalesce to a generalised erythematous rash with atypical target lesions, progressing to vesicles and bullae extensive necrotic skin lesions that fall off in large sheets.50,51 When the main finding is erythema, Nikolsky's sign can guide the diagnosis, although not exclusively for SJS/TEN. This sign is an epidermal detachment caused by applying a tangential pressure on an erythematous area without blisters.21
Mucosal involvement occurs in approximately 90% of cases of SJS/TEN and may precede or follow the rash.52 Virtually all patients have ulcers, painful and haemorrhagic crusts and erosions which vary in severity on any mucosal surface. Stomatitis and mucositis affect oral intake with consequent dehydration and malnutrition. In addition to the oral mucosa, ocular mucosa, with purulent conjunctivitis and inflammation, nasal, pharyngeal, respiratory and digestive tract, anogenital mucosa may also be involved, resulting in potentially life-threatening complications, such as bleeding and infection.20,25 Ocular involvement occurs in approximately 80% of patients. Urethritis that develops in two-third of cases can cause urinary retention. Pharyngeal mucosa is affected in almost all patients, the least affected being tracheal, bronchial and oesophageal.53,54 Patients with very severe disease may develop renal and/or hepatic failure.20 Anaemia and lymphopenia are common in SJS/TEN.55 In severe cases hypoalbuminaemia, electrolyte disturbances and increased urea nitrogen and glucose may occur, due to the massive transdermal loss of fluid and the hypercatabolic state. Urea nitrogen of >10mmol/L and glucose >140mg/dL are considered markers of severe disease.56 In severe cases with extensive skin detachment, complications include acute massive fluid loss through the denuded skin with electrolyte disturbances, hypovolaemic shock, renal failure, bacteraemia, insulin resistance, hypercatabolic state and multiple organ dysfunction syndrome.57 There is a high risk of bacterial infection, with sepsis and septic shock, especially Staphylococcus aureus and Pseudomonas aeruginosa being the leading causes of death in patients with SJS/TEN.57
AGEP is a serious adverse skin reaction, considered type IV d hypersensitivity.58 Its incidence is about 1–5 cases per million population per year. It has been reported rarely in children and is usually related to viral and bacterial infections.59 When drugs are involved, penicillin, cefixime, clindamycin, vancomycin, macrolides are the most common agents.60 It presents with an acute onset of asymptomatic or mildly pruritic erythema, with hundreds of non-sterile follicular pustules, less than 5mm in diameter, frequently accompanied by fever and fatigue. In very rare cases children can have overlapping findings of other severe reactions, such as DRESS (facial oedema) or SJS/TEN (some epidermal detachment).6,59
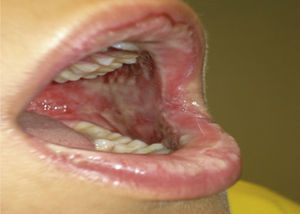
Case report 1Male, 11 years of age, presenting at 8–12h of finishing a week of treatment with amoxicillin/clavulanate for acute otitis media with fever, symptoms consisting of canker sores of the oral mucosa and lips without other accompanying symptoms. At 24h the fever and mucosal lesions appeared worse, so he went to urgent care, where he was treated with paracetamol, oral antiseptic+corticosteroid and rinses.
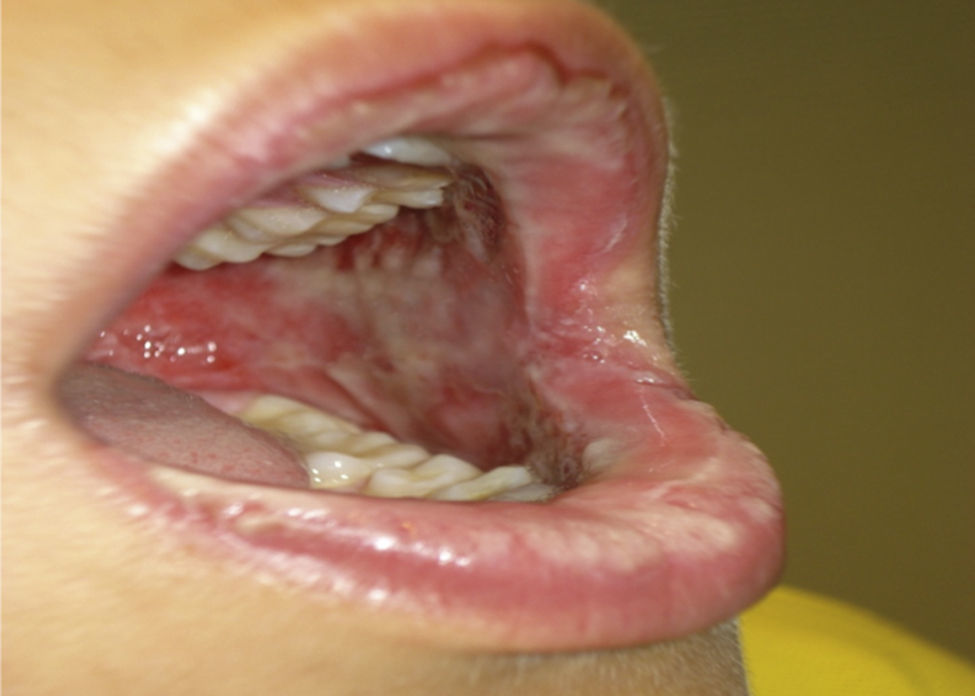
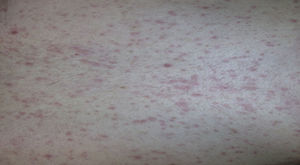
Two days later, due to clear worsening of the lesions (see Fig. 1), he went to urgent care where Herpes virus was ruled out after performing oral mucosa smear. Given the lack of improvement in the patient with oral mucosa lesions that prevented the intake of both solid and liquid, and isolated appearance of bullous lesions on the face, knee and leg, hospital admission was decided (at 5–6 days after the end of antibiotic treatment). Biopsy of lesions and lymphoblastic transformation test (LTT) were performed. Treatment was initiated with methylprednisolone 1mg/kg weight IV, absolute diet, fluid therapy and antihistamines, despite which there was overall worsening of his condition and of lesions extending to the trunk, genitals, and pharynx, with intense photophobia and ocular erythema.
He progressed well, with progressive improvement of the lesions in the following days, being discharged to home with corticosteroid treatment in decreasing doses at 7 days after admission.
Two years earlier he had presented a similar episode but with less involvement, which had occurred 5 days before oral sores and lesions on the lips, entering another hospital for 12 days. During admission he received treatment with amoxicillin/clavulanic acid, erythromycin and prednisolone, being diagnosed with “Stevens Johnson Syndrome in relation to alleged taking ibuprofen.” The mother doubted its involvement since the medication was not taken continuously.
Two months after this first episode he was readmitted for similar symptoms, after taking ibuprofen, carbenoxolone gel and topical Acyclovir for new aphthous lesions, remaining in hospital for 11 days until complete resolution of symptoms, receiving during this hospitalisation amoxicillin clavulanic acid, erythromycin and prednisolone.
From the second episode to admission to our hospital, the patient had not been treated with amoxicillin or amoxicillin clavulanic acid, although had occasionally taken ibuprofen.
Complementary studies showed the following results:
Basic blood tests, chest X-ray, IgG, M and A and tryptase serum were normal. Serology for EBV, VZV, HSV, toxoplasma and mycoplasma pneumoniae were negative. HBV, HCV, VHS-6 and herpes virus culture on cutaneous and oral lesions were negative on three occasions.
The result of skin biopsy was “Band-like lichenoid infiltrate with keratinocyte necrosis in dermoepidermal junction” confirming the suspected diagnosis of SJS.
LTT was positive for ibuprofen in acute phase, although not conclusive. It was repeated at 3 months of the reaction, yielding a stimulation index of less than 3 to amoxicillin/amoxicillin clavulanate, no repeated positivity for ibuprofen.
Immunoallergic study:
Total IgE normal and IgE specific to penicilloyl G, V, amoxicillin and ampicillin negative. PT to usual concentrations negative reading at 48 and 96h to amoxicillin, amoxicillin clavulanate (5%) and ibuprofen (10%). Skin tests (prick test) negative for amoxicillin, amoxicillin clavulanate, PPL, MDM and penicillin G. The patient and his mother refused to perform intradermal skin tests.
Five months after hospitalisation he presented a new outbreak without being on medication, initially very mild, with some oral mucosa vesicle, but with progressive worsening requiring admission 4 days later, receiving treatment with corticosteroids (intravenous methylprednisolone 1–1.5mg/kg) and paracetamol.
During this admission a study of lymphocyte subpopulations was conducted, and the proliferative response to mitogens PHA, Con A and PWM was performed with normal results. Herpes virus genomic amplification: PCR for Varicella zoster virus/HSV I and HSV II negative.
Despite the negativity in serology and smears for herpes virus treatment was given with oral velaciclovir for 6 months.
A new LTT at 8–12 weeks was proposed, but the patient and his mother refused any test, not presenting to the scheduled reviews.
Stevens Johnson syndrome was diagnosed (four episodes, three of them possibly related to taking amoxicillin/amoxicillin clavulanic acid and ibuprofen) and total avoidance was recommended.
Case report 2A three and a half year old male patient being treated with amoxicillin clavulanic acid and ibuprofen during a respiratory infection due to Moraxella catarrhalis who, after 48h of starting treatment, presented conjunctivitis and purulent rhinorrhoea with facial erythema and itching, worsening at 48h, with involvement of oral and labial mucosa treated as herpetic gingivostomatitis with acyclovir.
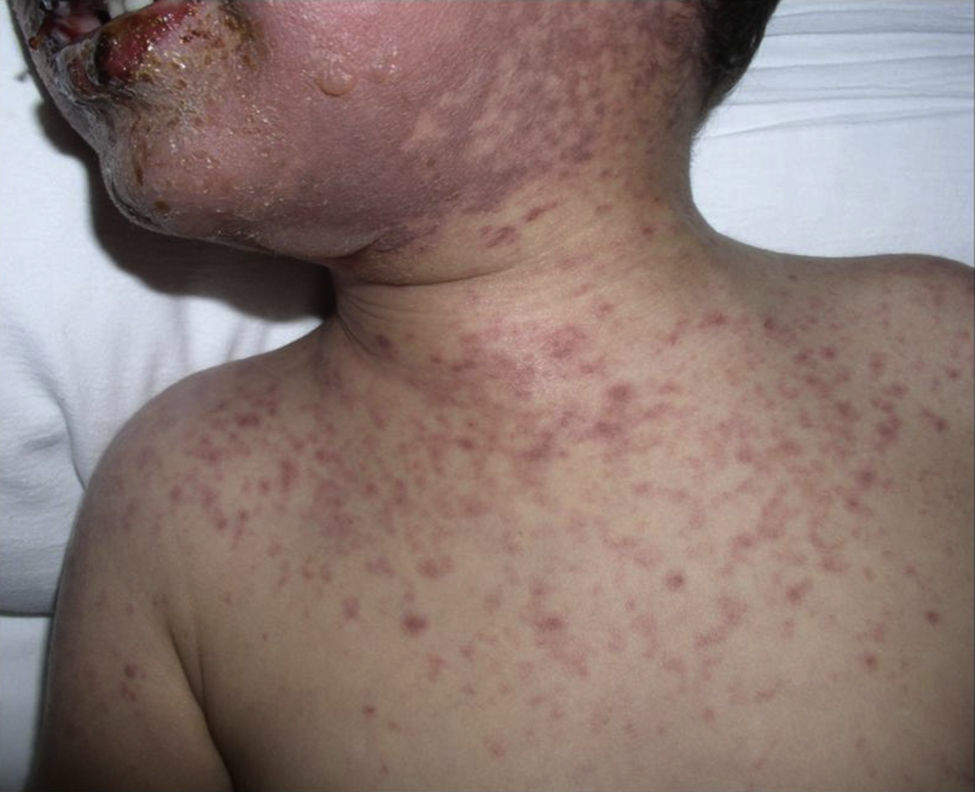
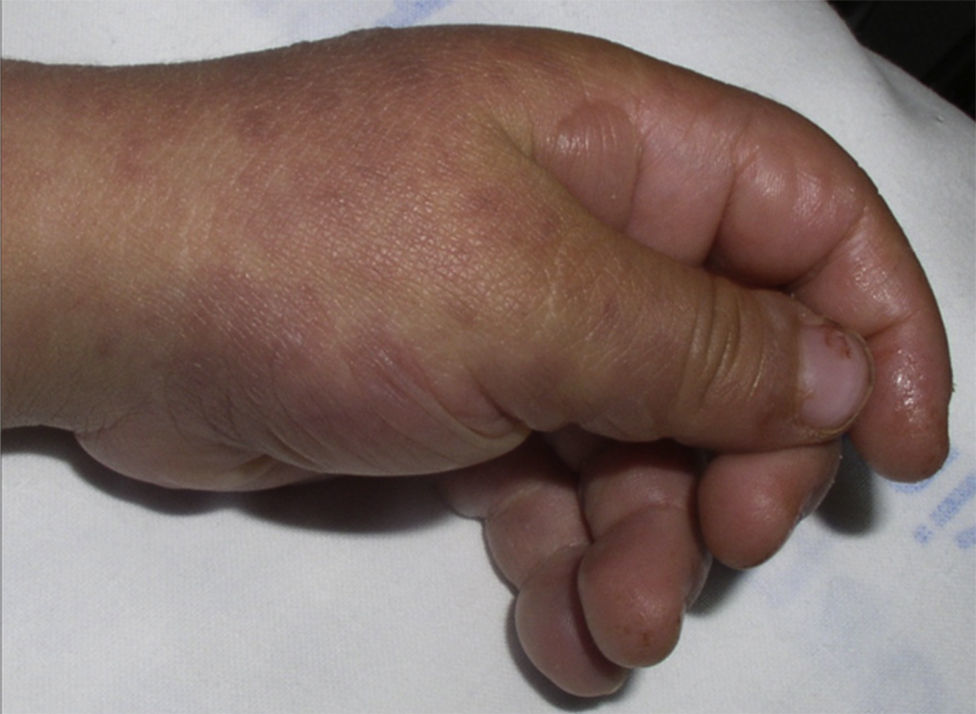
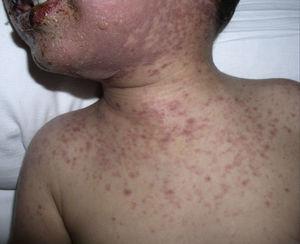
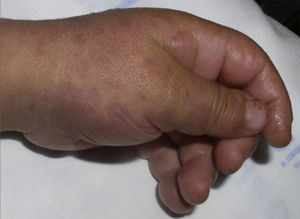
The patient required hospital admission 24h later due to presenting flaccid bullous lesions on the face, palms and soles, with target lesions in the trunk, and a positive Nikolsky sign (Figs. 2–3).
Methylprednisolone IV therapy was initiated at a dose of 2mg/kg weight, fluid therapy and routine haemodynamic support measures with analgesia (fentanyl and paracetamol) and antibiotic prophylaxis with erythromycin. Due to initial worsening of the lesions, with generalisation and greater involvement of oral and genital mucosa, admission to the ICU was considered, although it was finally not necessary since lesion progression remitted 48h after starting corticosteroid treatment. After gradual improvement without sequelae (except residual foreskin injury with mild stenosis), the patient was discharged with oral methylprednisolone in decreasing doses until completion in 14 days.
The following additional studies were conducted with the following results:
Routine blood tests, immunological study and basal serum tryptase normal. Blood cultures (three samples) negative. Serology negative for Hepatitis, Mycoplasma, Coxiella, Chlamydia, CMV, EBV, and Herpes virus, including herpesvirus 6, and genomic amplification of herpes virus in blood. Chest XR normal.
The result of skin biopsy was “Epidermal necrosis leading to subepidermal vesicle. Abundant apoptotic keratinocytes,” which confirmed suspected TEN.
Basal LTT performed during hospitalisation: inconclusive.
Immunoallergic study (determination at 8 weeks):
Total IgE normal. IgE specific to penicilloyl G, V, amoxicillin and ampicillin negative. Skin tests (prick test) negative for PPL, MDM, penicillin G, amoxicillin and amoxicillin clavulanate. Intradermal skin tests (ID) were not performed.
PT was negative reading at 48 and 96h for amoxicillin and amoxicillin clavulanic acid (5%), and ibuprofen (10%).
LTT performed at 12 months from admission was positive for amoxicillin and ibuprofen (over 3). Doubtful for amoxicillin and clavulanic acid (2.5).
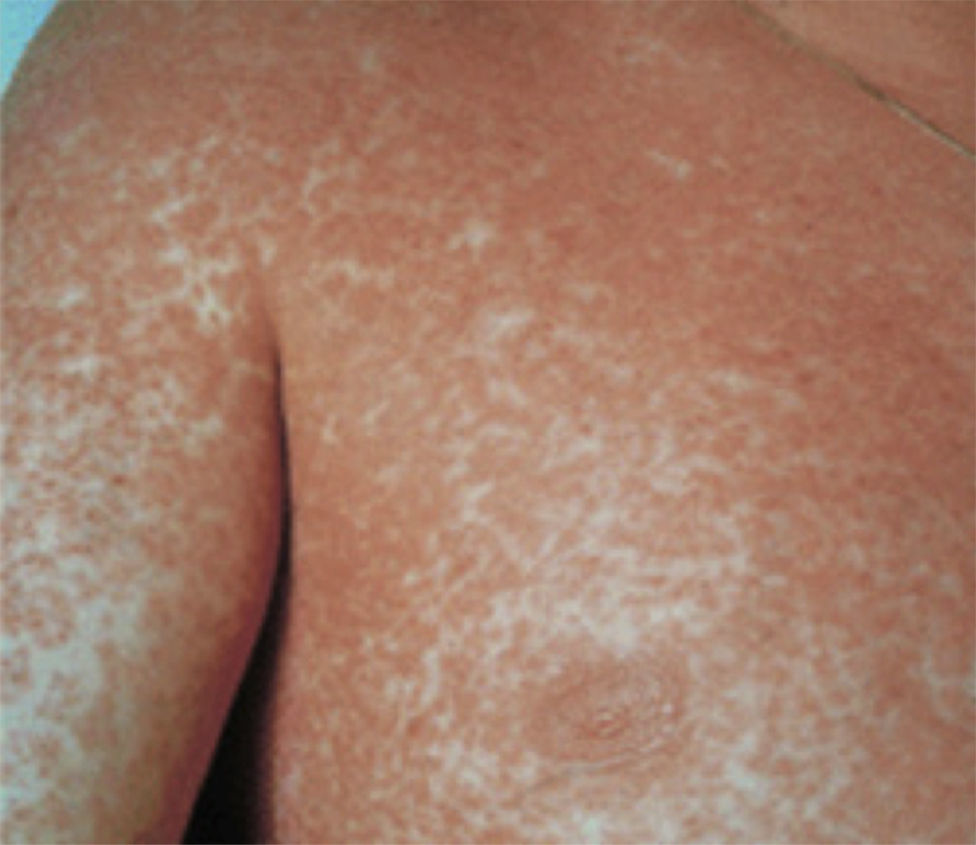
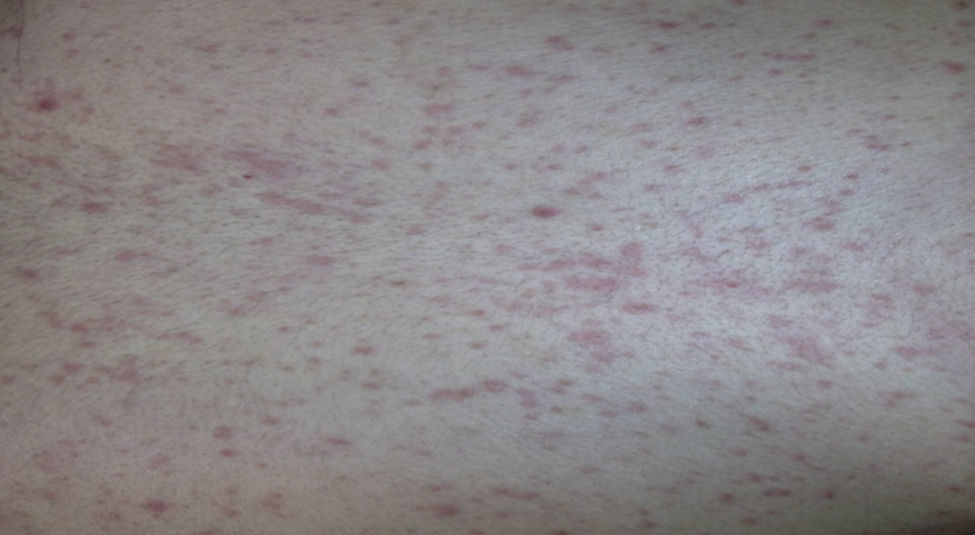
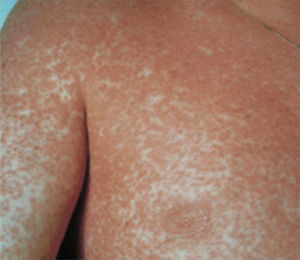
Case report 3A 6-year-old girl admitted for urticarial rash on face, trunk and limbs with high fever, of 3–4 days duration (Fig. 4). Six weeks before the rash the patient had been hospitalised for epidural abscess secondary to ethmoid sinusitis being treated with amoxicillin+clavulanic acid due to the lack of response with meropenem. Four weeks before the rash she had started treatment with phenytoin for status epilepticus secondary to abscess.
Due to the onset of rash meropenem and phenytoin were withdrawn and replaced by linezolid and levetiracetam respectively, beginning treatment with corticosteroids (methylprednisolone) IV (1mg/kg weight) and antihistamine.
After initial improvement, on the fourth day the patient presented clear worsening of rash with involvement of soles, abdomen, back, thighs, and generalised pruritus (Fig. 5). Levetiracetam was removed and the corticosteroid dose increased to 1.5mg/kg body weight.
The analysis revealed eosinophil count of 2100/μL without alterations in hepatic or renal function.
The patient was discharged 11 days later due to improvement with oral corticosteroids in descending pattern and antihistamine. A few hours later, fever and dysphagia began, progressing in the next 48h, so the patient was admitted again, observing right laterocervical lymphadenopathy, geographic tongue, high fever and increased serum creatinine and CRP with eosinophilia (9.5%), so corticosteroid IV treatment was resumed at doses of 1.5mg/kg.
Despite treatment, the patient presented progressive deterioration of renal function, with elevated cystatin C (1.74mg/L. Standard value: 0.62–1.11mg/L) with creatinine 0.74mg/dL, with an alpha-1 microglobulin of 16mg/24h, 24h timed microalbuminuria at 36μg/min and 24-h proteinuria of 221mg, reaching a maximum of 12.9% eosinophilia (at 18–20 days of initiation of rash). Six days after initiation of treatment symptoms improved, with normalisation in the number of eosinophils and creatinine, so the patient was discharged 7 days after admission, with steroids in descending pattern.
Neither LTT nor skin biopsy could be performed due to the girl's anxiety and psychological rejection, and parental refusal.
At 2 weeks after discharge during analytical monitoring control a count of 850 eosinophils were seen without liver involvement, creatinine 0.73mg/dL, cystatin C 1.12mg/L and decrease in the basal cortisol with iatrogenic Cushing (involvement of face and trunk), so corticosteroid dose was reduced slowly and progressively.
At 8 weeks after discharge from the second admission, total resolution of skin symptoms was observed, with clear improvement of Cushing and renal function (cystatin C 0.91mg/L), with a decrease in the number of eosinophils, suspending treatment at 10 weeks.
Two weeks later the patient presented mild sinusitis that was treated without incident with amoxicillin/clavulanate.
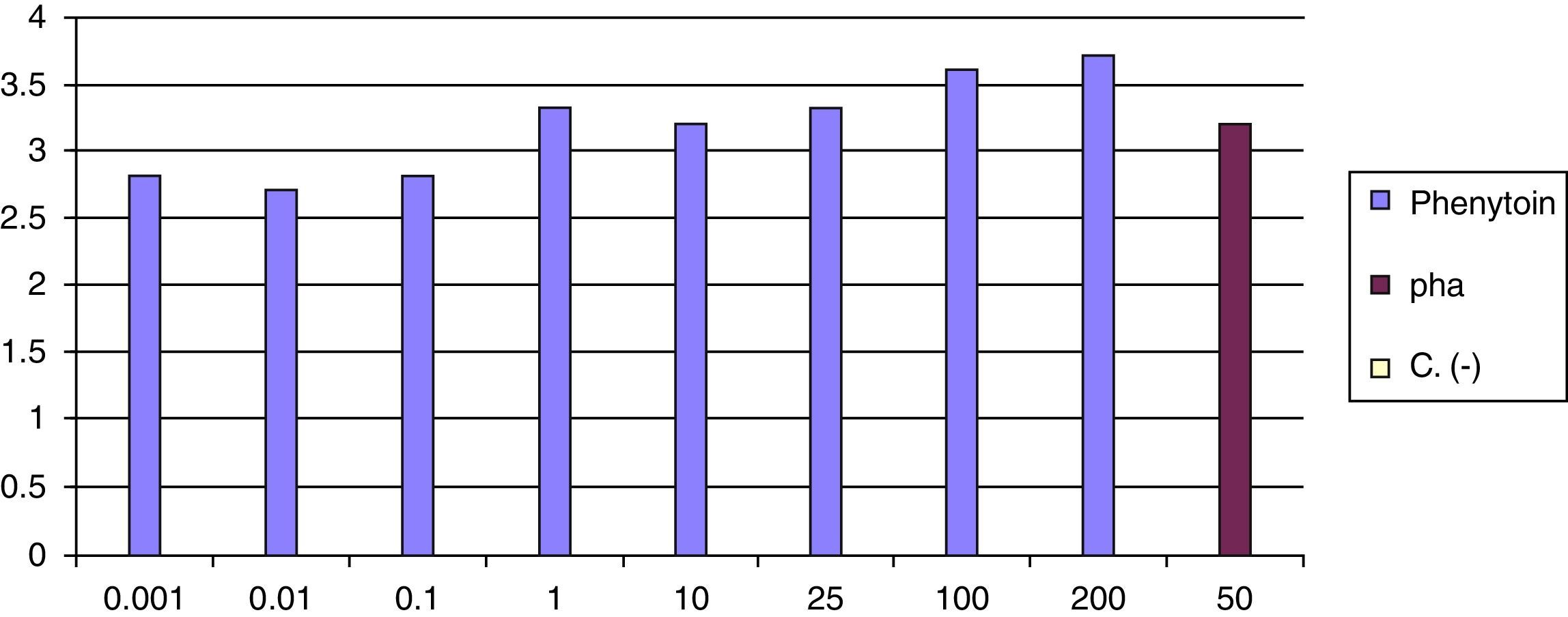
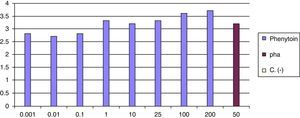
At 8 weeks of the withdrawal of corticosteroids LTT was performed, which was positive for phenytoin (greater than 3 result) (Fig. 6).
DRESS associated with phenytoin was diagnosed, with avoidance of other anticonvulsants advised, except benzodiazepines and valproate.
Discussion (diagnostic and therapeutic aspects)AGEPBesides the well-differentiated clinical features of this disease described above, the histological findings confirm the diagnosis, and include: subcorneal pustules with neutrophils, with marked dermal oedema and perivascular infiltrates with occasional neutrophils.6 Eosinophils can also be seen in the superficial dermis and focal necrosis of keratinocytes.61
Removal of the causal factor is the main treatment, with spontaneous resolution in 1–2 weeks with a pattern of “punctata” flaking. No scars develop or are limited. Antihistamines can be used for itching, and a short course of oral corticosteroids could be useful.6,62
SJS/TENThe diagnosis of SJS/TEN is considered in relation to drugs if the patient is exposed to the drug within 8 weeks of the start of the rash. It will be considered of infectious origin if such a process is presented in the week prior to the rash, and if IgM to infectious agents can be demonstrated.30,31 It has been seen that the latency time between the introduction of the drug and the start of SJS/TEN varies depending on the drug, being much shorter in the case of antibiotics and sulphonamides,44 even days, as in the case of a 6-year-old girl who developed TEN after 5 days of receiving procaine penicillin and 2 days ceftriaxone,45 In both cases presented here, the interval in the first one was 7–8 days and in the second was 48–72h. Sometimes both infectious agents and drugs are identified as potential precipitants of the disease. It is biologically plausible that the interaction between the infectious agent and the drug or its metabolite can precipitate severe skin reactions. In this case, the infection probably acts as a cofactor.30 In the first case it is possible that the lack of consistency in relation to the intake of drugs in the first symptoms, where there is only a suspicion of ibuprofen, is because the main factor has been the infection and the drug, a cofactor. Interestingly the LTT was positive in the symptomatic phase and then negative, possibly because there was no infection at that time. The largest cohort published by Levi et al., including 80 patients under 15 years, concluded that the sulfonamides and anticonvulsants (phenobarbital, lamotrigine and carbamazepine) were the most frequent causative agents,28 but as mentioned above, others such as antibiotics and NSAIDs can also be the cause.11,28,30,31 Moreover, in many cases of children with SJS/TEN, more than one drug is considered suspect, so it is better to use the term “suspected” rather than “causal”.28,31 In the cases of SJS and TEN presented here two “suspected” drugs were involved.
There are no universally accepted diagnostic criteria for STS/TEN, and histological findings are neither specific nor diagnostic. Despite these limitations, the diagnosis of SJS or TEN would be appropriate in a patient with the following findings63:
- •
A history suggestive of exposure to drug or disease. Drug exposure commonly precedes the onset of symptoms within 1–4 weeks (average 14 days), but re-exposure can result in the onset of symptoms in as little as 48h interval.64
- •
A prodrome of febrile illness and discomfort.
- •
Erythematous macules, target lesions, or diffuse erythema progressing to vesicles and blisters.
- •
Positive Nikolsky sign and/or sign of spread of blister.
- •
Oral, genital or ocular mucositis with painful mucosal erosions.
- •
Necrosis and detachment of the epidermis of variable degree.
The differential diagnosis in children should be made particularly with: Erythema multiforme, drug-induced erythematous and erythrodermic rash, AGEP, staphylococcal scalded skin syndrome and linear IgA bullous dermatosis.
The diagnosis of SJS/TEN is primarily clinical, and is confirmed by skin biopsy with the above findings,24 as in the two cases presented here.
The aetiological diagnosis of severe non-immediate reactions is difficult, and the value of current allergological tests is not well defined in these cases. Diagnosis is based on clinical history, the empirical risk of drugs to trigger SJS/TEN, and in vivo and in vitro tests of suspected drugs.65 A detailed history of all new medications taken during the 8 weeks before the onset of the disease will be obtained, although the most important period to consider is 1–2 weeks before.20 The challenge test is contraindicated because rechallenge may trigger a new episode of greater severity.66
Following the ESCD algorithm67 for evaluating allergological non-immediate reactions to beta-lactams, the PT is used with antibiotics or other drugs with 5% petrolatum/Vaseline. The PT is used from the commercial form and is safer than the intradermal test, as there are reports that the intradermal test itself could trigger SJS/TEN.25 The sensitivity varies depending on the vehicle used, the type of rash (higher in maculopapular rash and the AGEP, and lower in SJS and TEN); and higher in beta-lactam antibiotics, anticonvulsants and others.67–69 The sensitivity of PT in SJS/TEN is low (10.8–37.5%).70 Late reading of intradermal test appears to be more sensitive than the PT,65 with a negative predictive value of about 90%,71 but is also less specific25,67 and is uncertain.25
LTT attempts to quantify the activation of T cells in response to the drug. It has been shown useful in the diagnosis of delayed hypersensitivity reactions, but in patients with SJS/TEN the sensitivity is low.25,68,69 The LTT should be made within 1 week of the start of the rash.72 In Case 1 it was doubtfully positive in acute ibuprofen, and in Case 2 interestingly, it was negative in the acute phase, being instead clearly positive at 12 months to amoxicillin and ibuprofen and doubtful to amoxicillin+clavulanic.
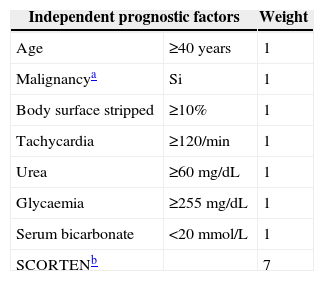
Patients suspected of having STS or TEN must be admitted to hospital. As soon as the diagnosis is established the severity and prognosis of the disease should be quickly assessed to define appropriate management.73 The prognosis of individual patients can be evaluated quickly using the so-called SCORTEN system.74 This system is based on seven clinical and laboratory variables easily measurable (Table 1) and has been validated for use between 24 and 72h of hospitalisation for SJS/TEN.75,76 The decision to admit the patient to intensive care or the burn unit should be taken case by case, based on the extent of skin involvement and the presence of comorbidities. Patients with limited skin involvement with SCORTEN score of 0–1, and not rapidly progressive disease can be treated in regular hospital units.77 Patients with more severe disease and a SCORTEN≥2 should be transferred to intensive care units or burn units if possible. It is emphasised that this score has not been validated yet in childhood, but may be useful.20 For patients with suspected drug-induced SJS/TEN, early identification and removal of the offending agent may improve the prognosis.57
The main assumptions of supportive care are the same as for large burns, and include wound care, management of fluid and electrolyte balance, nutritional support, eye care, temperature control, pain management and monitoring or treatment of superinfections.56,66,78
There is no unanimous agreement on the optimal procedure for the management of wounds or injuries, and different strategies are used in different centres.79–81
Recovery may be slow, taking several weeks and maybe even longer in patients with TEN. There can be sloughing mucous areas and hair, hypo- or hyperpigmentation, alopecia, anonychia, chronic ulcers or strictures in mucosal areas, and even visual decline or blindness.34
General care measures include care by multidisciplinary teams, medical equipment and nursing, specialising in the management of extensive skin and mucosal stripping. Full care includes eye examinations and supportive care, with special emphasis on injury management, fluid balance, nutritional and respiratory support, aggressive septic monitoring, pain management, and others.82–86 Adequate nutritional support is necessary, especially considering that the requirements of children with SJS/TEN are increased, which is why an additional 30% over the basal need is calculated.87
The treatment of wounds is important to avoid complications arising from the loss of barrier function, and although there is some debate, the usual tendency is to practice a gentle debridement of broken blisters, remove the necrotic epithelium, topical treatment with antimicrobials and cover the wound with dressings, whether biological or biosynthetic.86 Antiseptic solution dressings, silver sulfadiazine type (if not caused by sulfonamides), chlorhexidine or silver nitrate86 shall be applied.
The literature on specific treatment in children is scarce and controversial, with the absence of clinical trials, and observational studies with only a small number of patients.20 In general, there are two major trends in specific treatment in children, (IVIG) and corticosteroids.20 IVIG had shown some promising effects for inhibiting FasL (Fas ligand)-mediated keratinocyte apoptosis, by blocking Fas receptors20; however, as stated above, it is now accepted that the most important mediator is granulysin.31 Many studies have shown that when IVIG is used in doses of 2–4g/kg, within the first 4 days of the onset of rash, it shortens both the time of cessation of disease progression, as well as the time required for complete re-epithelialisation.88–90
A European multicentre study suggested that a short course of moderate to high doses of systemic corticosteroids (prednisone 1–2mg/kg/day for 3–5 days) may not be harmful and have a beneficial effect if given early in the course of the disease (within 24–48h of onset of symptoms).91 However, an analysis of the mortality of the RegiSCAR cohort and a systematic review of case series do not confirm a survival benefit for patients treated with systemic corticosteroids.92,93
The review of studies with corticosteroids does not clearly indicate the use of systemic corticosteroids for the treatment of SJS/TEN. Moreover, since theoretically corticosteroids increase the risk of sepsis and protein catabolism and decrease the rate of re-epithelialisation, its use in patients with extensive stripping of skin would be contraindicated.94 A recently published meta-analysis of SJS/TEN in children found that patients treated with supportive care alone had higher mortality and morbidity rates, and that patients treated with the combination of corticosteroids and IVIG appeared to have better outcomes.95
Finally, in terms of recurrences observed in Case 1 they are in line with findings in the literature, since one in five cases of a case series in childhood presented recurrences, in some cases multiple, denoting the possibility of a vulnerability and potential genetic predisposition.96
DRESSGiven the high variability in the clinical presentation of DRESS, diagnosis often requires a high index of suspicion and clinical judgement.
In typical cases the diagnosis is based on the following findings15:
- •
History of exposure to high-risk medications, such as allopurinol or antiepileptic drugs in 2–6 weeks before the onset of symptoms.
- •
Progressing confluent morbilliform rash and exfoliative dermatitis or infiltrated erythema affecting >90% of the total body surface.
- •
The extent of the affected body surface can be calculated similarly to burns, for example, with the rule of nines or the palm method.15
- •
Haematological abnormalities (eosinophilia >700/μL and/or atypical lymphocytosis).
- •
Systemic symptoms and organ involvement, which may include:
- •
Fever (38–40°C)
- •
Enlarged lymph nodes
- •
Altered liver function test.
- •
Renal involvement.
- •
Interstitial pneumonia and/or pleural effusion.
- •
Myocarditis.
- •
The diagnosis is uncertain in patients with atypical presentations including16:
- •
Unclear relationship with exposure to a drug.
- •
Absent or transient skin rash
- •
Absence of eosinophilia.
- •
Mild or absent systemic symptoms.
- •
A possibility, although rare, is the diagnosis of DRESS without the “D” (no clear causal agent), without “R” (rash is sometimes absent), without the “E” (absent eosinophilia in 10–50% of cases), or even with no systemic symptoms.96
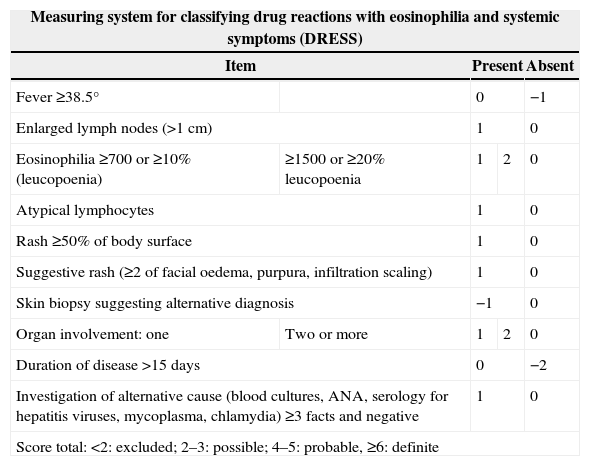
To help clinicians confirm or exclude the diagnosis of DRESS, the European Register of Severe Cutaneous Adverse Reactions (RegiSCAR) developed a measurement system based on clinical findings, extent of affected skin, organ involvement and clinical course.13,97 DRESS is classified according to the result as defined, probable or possible (Table 2).
Measuring system for classifying DRESS syndrome.
| Measuring system for classifying drug reactions with eosinophilia and systemic symptoms (DRESS) | ||||
|---|---|---|---|---|
| Item | Present | Absent | ||
| Fever ≥38.5° | 0 | −1 | ||
| Enlarged lymph nodes (>1cm) | 1 | 0 | ||
| Eosinophilia ≥700 or ≥10% (leucopoenia) | ≥1500 or ≥20% leucopoenia | 1 | 2 | 0 |
| Atypical lymphocytes | 1 | 0 | ||
| Rash ≥50% of body surface | 1 | 0 | ||
| Suggestive rash (≥2 of facial oedema, purpura, infiltration scaling) | 1 | 0 | ||
| Skin biopsy suggesting alternative diagnosis | −1 | 0 | ||
| Organ involvement: one | Two or more | 1 | 2 | 0 |
| Duration of disease >15 days | 0 | −2 | ||
| Investigation of alternative cause (blood cultures, ANA, serology for hepatitis viruses, mycoplasma, chlamydia) ≥3 facts and negative | 1 | 0 | ||
| Score total: <2: excluded; 2–3: possible; 4–5: probable, ≥6: definite | ||||
Since the score incorporates some information that is only obtained later in the course of the disease (e.g. longer than 15 days or poorer results of laboratory parameters over time), it is more useful in the retrospective validation than in early diagnosis of DRESS.15
Histopathological analysis of the skin can help confirm the diagnosis of DRESS.12 The most common finding is a dense perivascular lymphocytic infiltrate in the papillary dermis, eosinophils, and dermal oedema,5 and it is generally thicker than that observed in other serious skin reactions.4
Diagnostic tests can be used for delayed reactions, such as PT and LTT, to try to identify the causative drug. Because in acute immunosuppression skin reactivity may decrease, or there may be false positives while the skin is still excited, the optimal time to carry it out must be at least 6 weeks after resolution of symptoms.98 In the study by Santiago et al., 72% of suspected cases of carbamazepine-induced DRESS had a positive result in the PT, while with phenytoin, the test was positive only in 14.3% of cases,99 although in another study the results were better with phenytoin (PPV 60%).100 In our case PT was not performed. LTT is not well standardised so it cannot be recommended for routine use,101 although it is considered as a valuable and potentially useful test when performed at the appropriate time according to the type of reaction, which in the case of DRESS seems appropriate when 5–8 weeks have elapsed after start of rash.75
The differential diagnosis in children should be done primarily with STS/TEN syndrome and AGEP.15
In the treatment of DRESS it is of the utmost importance to identify and promptly remove the offending drug. It is also suggested not to introduce new drugs during the course of the disease.15 Patients with exfoliative dermatitis require fluid, electrolytes and nutritional support.15,25
Patients without organ involvement and modest elevation of transaminases (<3 times normal) can be treated symptomatically with high- or very high-potency topical cutaneous corticosteroids, up to three times a day, for 1 week.15 In these cases it is unclear whether systemic corticosteroids may shorten the clinical course.
The use of systemic corticosteroids for the treatment of DRESS with severe organ involvement has not been evaluated in randomised trials, and it is unclear whether they shorten the clinical course, but there is general consensus among experts about their use, particularly in patients with renal and/or lung involvement. The optimal dose and duration of treatment are unknown,15 but moderate to high doses of oral corticosteroids are recommended (0.5–2mg/kg/day of prednisone or equivalent) maintained until clinical improvement and normalisation of laboratory parameters, with gradual withdrawal over the following 8–12 weeks, as more rapid withdrawal increases the risk of relapse.15
In observational retrospective studies, many patients with DRESS, with or without severe organ involvement, are treated with systemic corticosteroids.16,102,103 Systemic corticosteroids do not appear to be harmful in DRESS, or increase the risk of death, as has been demonstrated in a series of 60 patients.102 For other treatments, such as IVIG, there is no evidence of positive effects, so their use is not recommended.15
Many patients recover completely weeks to months after drug withdrawal. Since autoimmune diseases have been reported months or years after the resolution of DRESS,104,105 monitoring is recommended for their possible occurrence.15
In summary, we report three paediatric cases, one with recurrent SJS with involvement of ibuprofen and amoxicillin/amoxicillin clavulanic acid with positive LTT in the acute phase for ibuprofen without further confirmation; another of TEN, relative to amoxicillin clavulanic acid and ibuprofen with positive LTT to both drugs; and a case of DRESS secondary to phenytoin with positive LTT, with renal involvement (acute interstitial nephritis with moderate renal impairment).
Severe delayed skin reactions triggered by drugs are rare but potentially serious and a high index of suspicion based on clinical history is essential for diagnosis. Skin biopsy will confirm the clinical diagnosis while tests for delayed hypersensitivity, especially patch testing and the lymphoblastic transformation tests, may be important to confirm the aetiological diagnosis, in our cases emphasising the latter. All cases progressed well without sequelae and were treated with systemic corticosteroids as soon as the diagnosis was suspected.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of dataThe authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.
Conflict of interestThere is no conflict of interest for any autor.
Sofía Sanchez (Laboratory Technician Immunoallergy, Hospital La Paz, Madrid, Spain).