Atopic dermatitis (AD) is a chronic inflammatory pruritic skin heterogeneous disease, which does not have an objective tool able to permit comparison between patients and to monitor changes due to a specific treatment. Several assessment methods have been developed to evaluate the AD.
ObjectivesThe aim of this study was to investigate the agreement between two observers in the assessment of the severity of AD applying SCORAD index and EASI in infants and young children with AD. The variations of parameters that compose each score, as well as the inter-observer variation for both scores, were also studied.
MethodsWe evaluated 42 infants and young children admitted and followed in a paediatric allergy centre (UNIFESP-EPM). All children met the diagnostic criteria for clinical AD established by Hanifin and Rajka. Two investigators graded the severity of AD, applying the SCORAD index and EASI. The two scoring systems assessed the variation between baseline evaluation and one month after treatment.
ResultsSignificant correlations were observed comparing both scores for each physician and each evaluation. There were no differences between the mean SCORAD and EASI scores for each physician, in the two evaluations. There was a significant decrease in the mean total SCORAD and EASI score and its components, except for the item Upper Extremities EASI
ConclusionBoth scoring systems for AD are valid, reproducible and responsive in monitoring AD patients. Further studies will be necessary to investigate the development of AD and the best evaluation. SCORAD showed itself to be an excellent score to detect the development of AD, whereas the EASI are suitable to follow up in drug-effect studies of AD for research purposes.
Atopic dermatitis (AD) is a chronic inflammatory pruritic skin disease that affects a large number of children and adults in industrialised countries. The onset of AD generally occurs before the first 6months of life in 45 % of affected children, during the first year of life in 60 %, and before the age of 5years in at least 85 %. The degree of severity may suggest adequate therapeutic options, and may represent a risk factor for developing aeroallergen sensitisation and asthma1.
Overall 60 % of patients with severe AD, may develop asthma later in childhood2,3. Children with atopic eczema and with a similar pattern of sensitisation to that of wheezing children (ie, showing any of the less prevalent sensitisation types of wheat, cat, mite, soy, or birch sensitisation) were at increased risk of subsequent asthma2.
Especially when severe, AD can be extremely disabling, causing major psychological problems, and, in the case of a young child, be overwhelming for the entire family. Children with AD often have behavioural problems such as increased dependency, fearfulness and sleep difficulties4.
AD is a heterogeneous disease which does not have an objective tool able to permit comparison between patients and to monitor changes due to a specific treatment. Because of this, numerous scoring systems have been described to allow the study of AD and to evaluate its implication on quality of life. Several assessment methods have been developed to evaluate the severity of AD5–9.
Schmitt et al10 sought to assess the validity, reliability, sensitivity to change, and ease of use of outcome measurements for atopic eczema. They concluded that only SCORAD, EASI, and POEM perform adequately, therefore these scales should be used in clinical research and for clinical monitoring in future studies.
The European Task Force on AD has developed a composite severity index based on a broad consensus by dermatologists. The resulting SCORAD (Scoring Atopic Dermatitis) index combines objective symptoms (extent and intensity) and subjective criteria (daytime pruritus and sleep loss). It has been extensively tested in trials and validity, reliability, sensitivity, and acceptability testing of this scoring system has been widely published5.
The Eczema Area and Severity Index (EASI) is another composite index including an assessment of disease extent and percent of body surface area involved, converted to a proportional factor, in four body regions (head and neck, lower limbs, upper limbs and trunk)11. EASI was developed to be effective in assessing patients with mild to severe AD, both paediatric and adult therapeutic. This instrument was designed by modifying the general scheme used in the psoriasis area and severity index (PASI) as suggested by Haniffin et al11.
The current AD scoring system permits defining in numerical terms (objectively) the severity of the disease in a single patient in a more scientific way, and allows detecting changes due to treatment or disease worsening. It is not clear how to choose a certain scoring system for AD and up to now there has been no consensus on this subject, mainly in the scoring system for children.
The purpose of this study is to investigate the agreement between two observers in the assessment of the severity of AD applying the SCORAD index and the EASI in infants and young children with AD. The variations of parameters that compose each score, as well as the inter-observer variation for both scores, were also studied.
PATIENTS AND METHODSThe information needed to calculate the scores was easily obtainable by a clinician. We evaluated 42 infants and young children admitted and followed in a paediatric allergy centre (UNIFESP-EPM) in Sao Paulo, Brazil. All children met the diagnostic criteria for clinical AD established by Hanifin and Rajka11.
Two investigators graded the severity of AD, applying the SCORAD index5 and EASI11. The SCORAD index includes the assessment, by a physician, of objective signs (extent and intensity) and of subjective symptoms (pruritus and sleep disturbance) compiled on an analogue scale by the parents. Extent was calculated using the "rule of nine" and expressed the skin surface area involved. Items that were evaluated in the intensity criteria were erythema, oedema/ papulation, oozing/crusts, excoriations, lichenification, and dryness of involved skin (from 0 to 3 points for each item). The final score was then calculated using the following equation: A/5 + 7B/2 + C (A = extent; B = intensity; C = subjective symptoms). The SCORAD index range lies between 0 and 103. Based on the SCORAD index results, AD has been classified, as reported, into mild (< 25), moderate (25–50) and severe (> 50) forms12.
The EASI is also a composite index, including the assessment of disease extent and percent of involved body surface area, converted to a proportional factor (scale from 0 to 6, in four body regions (head and neck, lower limbs, upper limbs, and trunk). The proportion allocated to each body region depends on the patient's age. In patients older than 8years, proportions are 10 % for head and neck, 20 % for upper extremities, 30 % for trunk and 40 % for lower extremities; in patients aged less than 7years, proportions are 20 % for head and neck, 20 % for upper extremities, 30 % for trunk and 30 % for lower extremities. The EASI also includes an assessment of erythema (E), infiltration and_or papulation (I), excoriation (Ex) and lichenification (L), each on a scale from 0 to 3. The EASI's minimum score is 0 and the maximum is 72. The algorithm for calculating the EASI uses, for each body region, the sum of the clinical sign scores (E + I + Ex + L) multiplied by the body surface area, multiplied by the proportional factor.
The total EASI score is the sum of the four body-region scores13.
The two scoring systems assessed the variation between baseline evaluation and 1month after treatment. All AD children were given topical steroids: medium-dose momethasone or fluticasone twice daily. The topic steroids were applied on average 10 % of total body surface area in patients. Antihistamines (cetrizine or hydroxyzine) were used for relief of symptoms when needed. None of these children received systemic steroids during the study.
All parents and/or guardians have signed the informed consent. This study was approved by the Ethical Committee of Federal University of Sao Paulo-Escola Paulista de Medicina.
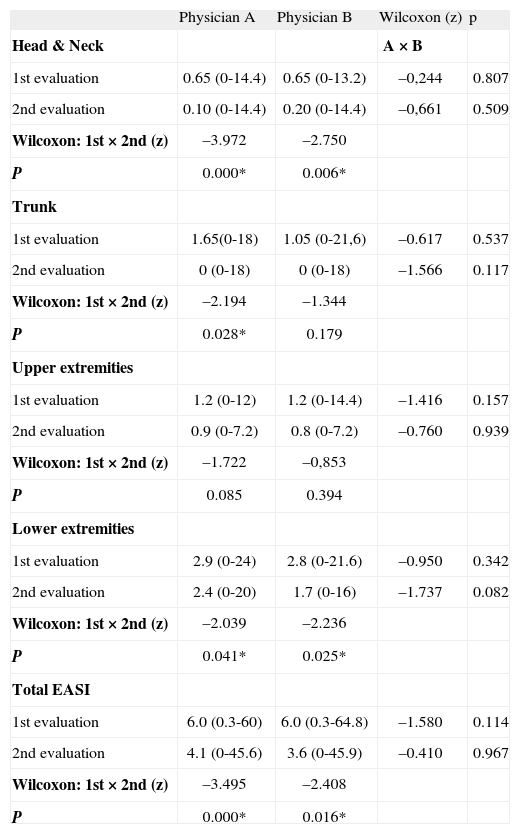
RESULTSThe mean EASI scores (total and components; range) given by physicians A and B, obtained in the two evaluations 4weeks apart are presented in Tabla I and IV. There were no differences between the mean scores for each physician, in the two evaluations considering total EASI and its components separately (Tabla I). However, when the comparison was made between both evaluations for each physician, there was a significant decrease on the mean total EASI score and its components, except for the item Upper Extremities (Tabla I). Evidence for adequate internal consistency were found for the EASI (Crombach's Alpha 0.944).
Comparison between mean Eczema Area and Severity Index (EASI) scores (total and segments; range) obtained in two evaluations 4weeks apart by two physicians (A and B)
| Physician A | Physician B | Wilcoxon (z) | p | |
| Head & Neck | A × B | |||
| 1st evaluation | 0.65 (0-14.4) | 0.65 (0-13.2) | –0,244 | 0.807 |
| 2nd evaluation | 0.10 (0-14.4) | 0.20 (0-14.4) | –0,661 | 0.509 |
| Wilcoxon: 1st × 2nd (z) | –3.972 | –2.750 | ||
| P | 0.000* | 0.006* | ||
| Trunk | ||||
| 1st evaluation | 1.65(0-18) | 1.05 (0-21,6) | –0.617 | 0.537 |
| 2nd evaluation | 0 (0-18) | 0 (0-18) | –1.566 | 0.117 |
| Wilcoxon: 1st × 2nd (z) | –2.194 | –1.344 | ||
| P | 0.028* | 0.179 | ||
| Upper extremities | ||||
| 1st evaluation | 1.2 (0-12) | 1.2 (0-14.4) | –1.416 | 0.157 |
| 2nd evaluation | 0.9 (0-7.2) | 0.8 (0-7.2) | –0.760 | 0.939 |
| Wilcoxon: 1st × 2nd (z) | –1.722 | –0,853 | ||
| P | 0.085 | 0.394 | ||
| Lower extremities | ||||
| 1st evaluation | 2.9 (0-24) | 2.8 (0-21.6) | –0.950 | 0.342 |
| 2nd evaluation | 2.4 (0-20) | 1.7 (0-16) | –1.737 | 0.082 |
| Wilcoxon: 1st × 2nd (z) | –2.039 | –2.236 | ||
| P | 0.041* | 0.025* | ||
| Total EASI | ||||
| 1st evaluation | 6.0 (0.3-60) | 6.0 (0.3-64.8) | –1.580 | 0.114 |
| 2nd evaluation | 4.1 (0-45.6) | 3.6 (0-45.9) | –0.410 | 0.967 |
| Wilcoxon: 1st × 2nd (z) | –3.495 | –2.408 | ||
| P | 0.000* | 0.016* |
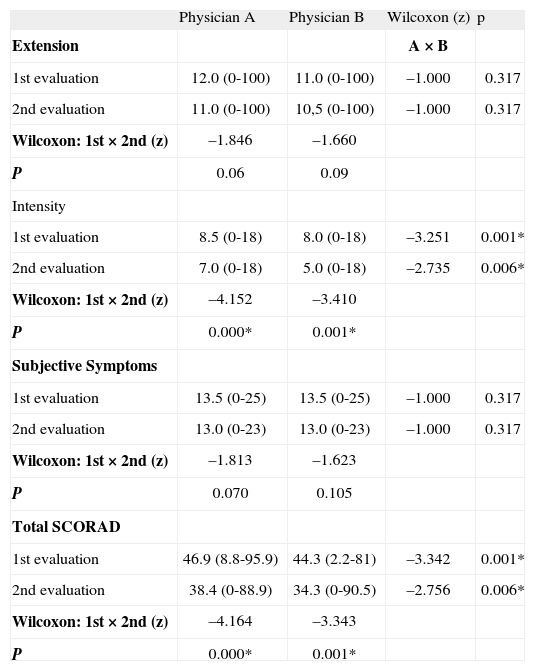
Comparison between mean Scoring Atopic Dermatitis (SCORAD) scores (criteria and total; range) obtained by two physicians (A e B) in two evaluations 4weeks apart
| Physician A | Physician B | Wilcoxon (z) | p | |
| Extension | A × B | |||
| 1st evaluation | 12.0 (0-100) | 11.0 (0-100) | –1.000 | 0.317 |
| 2nd evaluation | 11.0 (0-100) | 10,5 (0-100) | –1.000 | 0.317 |
| Wilcoxon: 1st × 2nd (z) | –1.846 | –1.660 | ||
| P | 0.06 | 0.09 | ||
| Intensity | ||||
| 1st evaluation | 8.5 (0-18) | 8.0 (0-18) | –3.251 | 0.001* |
| 2nd evaluation | 7.0 (0-18) | 5.0 (0-18) | –2.735 | 0.006* |
| Wilcoxon: 1st × 2nd (z) | –4.152 | –3.410 | ||
| P | 0.000* | 0.001* | ||
| Subjective Symptoms | ||||
| 1st evaluation | 13.5 (0-25) | 13.5 (0-25) | –1.000 | 0.317 |
| 2nd evaluation | 13.0 (0-23) | 13.0 (0-23) | –1.000 | 0.317 |
| Wilcoxon: 1st × 2nd (z) | –1.813 | –1.623 | ||
| P | 0.070 | 0.105 | ||
| Total SCORAD | ||||
| 1st evaluation | 46.9 (8.8-95.9) | 44.3 (2.2-81) | –3.342 | 0.001* |
| 2nd evaluation | 38.4 (0-88.9) | 34.3 (0-90.5) | –2.756 | 0.006* |
| Wilcoxon: 1st × 2nd (z) | –4.164 | –3.343 | ||
| P | 0.000* | 0.001* |
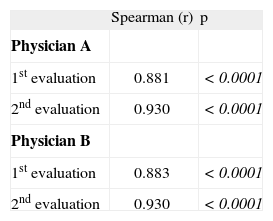
Correlation between Scoring Atopic Dermatitis (SCORAD) and Eczema Area and Severity Index (EASI) scores obtained by two physicians in two evaluations 4week apart
| Spearman (r) | p | |
| Physician A | ||
| 1st evaluation | 0.881 | <0.0001 |
| 2nd evaluation | 0.930 | <0.0001 |
| Physician B | ||
| 1st evaluation | 0.883 | <0.0001 |
| 2nd evaluation | 0.930 | <0.0001 |
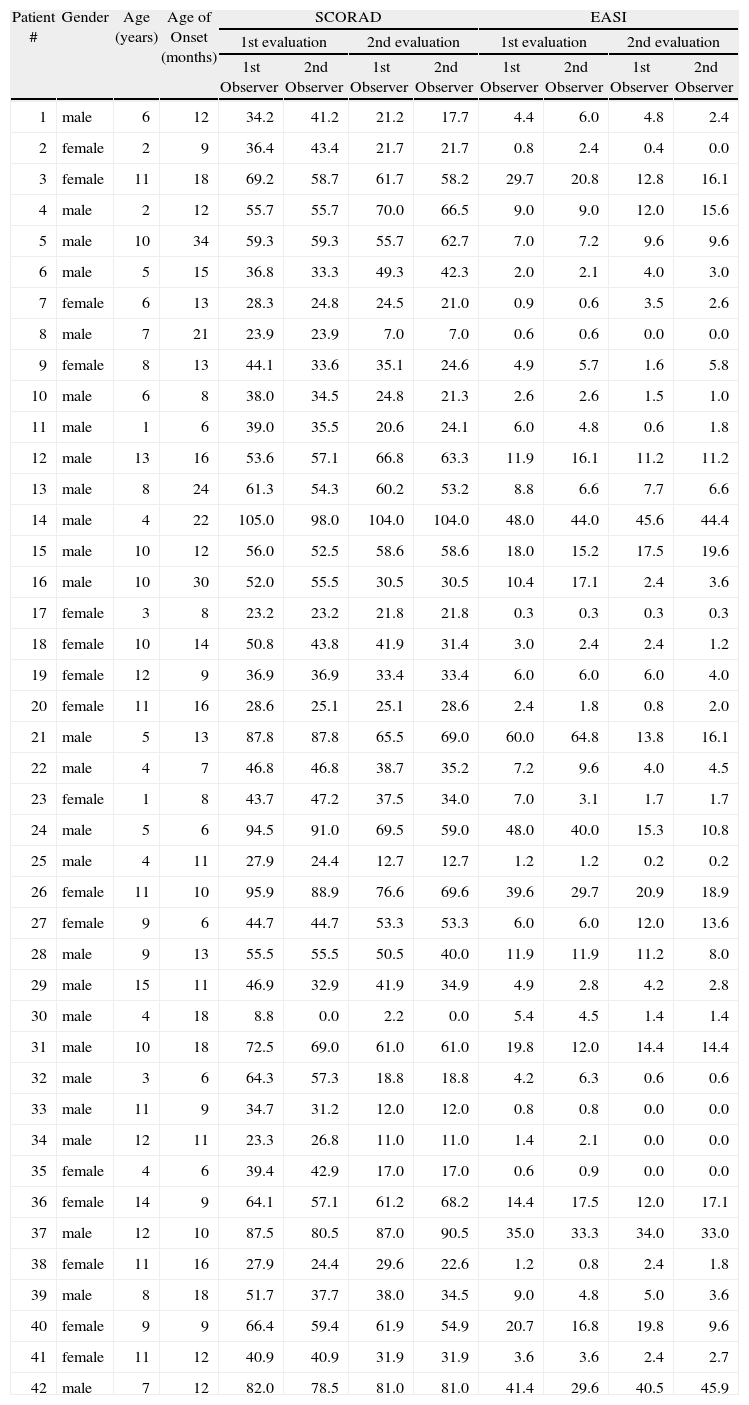
Features of children included in this study
| Patient # | Gender | Age (years) | Age of Onset (months) | SCORAD | EASI | ||||||
| 1st evaluation | 2nd evaluation | 1st evaluation | 2nd evaluation | ||||||||
| 1st Observer | 2nd Observer | 1st Observer | 2nd Observer | 1st Observer | 2nd Observer | 1st Observer | 2nd Observer | ||||
| 1 | male | 6 | 12 | 34.2 | 41.2 | 21.2 | 17.7 | 4.4 | 6.0 | 4.8 | 2.4 |
| 2 | female | 2 | 9 | 36.4 | 43.4 | 21.7 | 21.7 | 0.8 | 2.4 | 0.4 | 0.0 |
| 3 | female | 11 | 18 | 69.2 | 58.7 | 61.7 | 58.2 | 29.7 | 20.8 | 12.8 | 16.1 |
| 4 | male | 2 | 12 | 55.7 | 55.7 | 70.0 | 66.5 | 9.0 | 9.0 | 12.0 | 15.6 |
| 5 | male | 10 | 34 | 59.3 | 59.3 | 55.7 | 62.7 | 7.0 | 7.2 | 9.6 | 9.6 |
| 6 | male | 5 | 15 | 36.8 | 33.3 | 49.3 | 42.3 | 2.0 | 2.1 | 4.0 | 3.0 |
| 7 | female | 6 | 13 | 28.3 | 24.8 | 24.5 | 21.0 | 0.9 | 0.6 | 3.5 | 2.6 |
| 8 | male | 7 | 21 | 23.9 | 23.9 | 7.0 | 7.0 | 0.6 | 0.6 | 0.0 | 0.0 |
| 9 | female | 8 | 13 | 44.1 | 33.6 | 35.1 | 24.6 | 4.9 | 5.7 | 1.6 | 5.8 |
| 10 | male | 6 | 8 | 38.0 | 34.5 | 24.8 | 21.3 | 2.6 | 2.6 | 1.5 | 1.0 |
| 11 | male | 1 | 6 | 39.0 | 35.5 | 20.6 | 24.1 | 6.0 | 4.8 | 0.6 | 1.8 |
| 12 | male | 13 | 16 | 53.6 | 57.1 | 66.8 | 63.3 | 11.9 | 16.1 | 11.2 | 11.2 |
| 13 | male | 8 | 24 | 61.3 | 54.3 | 60.2 | 53.2 | 8.8 | 6.6 | 7.7 | 6.6 |
| 14 | male | 4 | 22 | 105.0 | 98.0 | 104.0 | 104.0 | 48.0 | 44.0 | 45.6 | 44.4 |
| 15 | male | 10 | 12 | 56.0 | 52.5 | 58.6 | 58.6 | 18.0 | 15.2 | 17.5 | 19.6 |
| 16 | male | 10 | 30 | 52.0 | 55.5 | 30.5 | 30.5 | 10.4 | 17.1 | 2.4 | 3.6 |
| 17 | female | 3 | 8 | 23.2 | 23.2 | 21.8 | 21.8 | 0.3 | 0.3 | 0.3 | 0.3 |
| 18 | female | 10 | 14 | 50.8 | 43.8 | 41.9 | 31.4 | 3.0 | 2.4 | 2.4 | 1.2 |
| 19 | female | 12 | 9 | 36.9 | 36.9 | 33.4 | 33.4 | 6.0 | 6.0 | 6.0 | 4.0 |
| 20 | female | 11 | 16 | 28.6 | 25.1 | 25.1 | 28.6 | 2.4 | 1.8 | 0.8 | 2.0 |
| 21 | male | 5 | 13 | 87.8 | 87.8 | 65.5 | 69.0 | 60.0 | 64.8 | 13.8 | 16.1 |
| 22 | male | 4 | 7 | 46.8 | 46.8 | 38.7 | 35.2 | 7.2 | 9.6 | 4.0 | 4.5 |
| 23 | female | 1 | 8 | 43.7 | 47.2 | 37.5 | 34.0 | 7.0 | 3.1 | 1.7 | 1.7 |
| 24 | male | 5 | 6 | 94.5 | 91.0 | 69.5 | 59.0 | 48.0 | 40.0 | 15.3 | 10.8 |
| 25 | male | 4 | 11 | 27.9 | 24.4 | 12.7 | 12.7 | 1.2 | 1.2 | 0.2 | 0.2 |
| 26 | female | 11 | 10 | 95.9 | 88.9 | 76.6 | 69.6 | 39.6 | 29.7 | 20.9 | 18.9 |
| 27 | female | 9 | 6 | 44.7 | 44.7 | 53.3 | 53.3 | 6.0 | 6.0 | 12.0 | 13.6 |
| 28 | male | 9 | 13 | 55.5 | 55.5 | 50.5 | 40.0 | 11.9 | 11.9 | 11.2 | 8.0 |
| 29 | male | 15 | 11 | 46.9 | 32.9 | 41.9 | 34.9 | 4.9 | 2.8 | 4.2 | 2.8 |
| 30 | male | 4 | 18 | 8.8 | 0.0 | 2.2 | 0.0 | 5.4 | 4.5 | 1.4 | 1.4 |
| 31 | male | 10 | 18 | 72.5 | 69.0 | 61.0 | 61.0 | 19.8 | 12.0 | 14.4 | 14.4 |
| 32 | male | 3 | 6 | 64.3 | 57.3 | 18.8 | 18.8 | 4.2 | 6.3 | 0.6 | 0.6 |
| 33 | male | 11 | 9 | 34.7 | 31.2 | 12.0 | 12.0 | 0.8 | 0.8 | 0.0 | 0.0 |
| 34 | male | 12 | 11 | 23.3 | 26.8 | 11.0 | 11.0 | 1.4 | 2.1 | 0.0 | 0.0 |
| 35 | female | 4 | 6 | 39.4 | 42.9 | 17.0 | 17.0 | 0.6 | 0.9 | 0.0 | 0.0 |
| 36 | female | 14 | 9 | 64.1 | 57.1 | 61.2 | 68.2 | 14.4 | 17.5 | 12.0 | 17.1 |
| 37 | male | 12 | 10 | 87.5 | 80.5 | 87.0 | 90.5 | 35.0 | 33.3 | 34.0 | 33.0 |
| 38 | female | 11 | 16 | 27.9 | 24.4 | 29.6 | 22.6 | 1.2 | 0.8 | 2.4 | 1.8 |
| 39 | male | 8 | 18 | 51.7 | 37.7 | 38.0 | 34.5 | 9.0 | 4.8 | 5.0 | 3.6 |
| 40 | female | 9 | 9 | 66.4 | 59.4 | 61.9 | 54.9 | 20.7 | 16.8 | 19.8 | 9.6 |
| 41 | female | 11 | 12 | 40.9 | 40.9 | 31.9 | 31.9 | 3.6 | 3.6 | 2.4 | 2.7 |
| 42 | male | 7 | 12 | 82.0 | 78.5 | 81.0 | 81.0 | 41.4 | 29.6 | 40.5 | 45.9 |
Tabla II shows data referring to the mean SCORAD index (total and components; range) given by the two physicians in two different evaluations. There were no significant differences between physicians comparing each evaluation, except for Intensity item and Total SCORAD. Similar results were observed comparing the mean scores obtained in the two evaluations for each physician (Tabla II). Evidence for adequate internal consistency were found for the SCORAD (Crombach's Alpha 0.974).
Significant correlations were observed comparing both scores for each physician and each evaluation
(Tabla III). Analysis comparing body surface area (BSA) measurements in the two scoring systems showed significant correlation (data not shown).
Statistical analysisSignificant differences of variables compared between two different evaluations for each physician were assessed by Wilcoxon test.
Correlations between SCORAD and EASI scores obtained by two physicians in two different evaluations were assessed by Spearman correlation test. Significant differences of BSA measurements compared between two scoring systems were assessed by Pearson correlation test. Internal consistence for EASI and SCORAD were assessed by Crombach's alpha. Statistical analysis was performed using a software program (SPSS for Windows version 12.0; SPSS Inc). Values of p < 0.05 were considered as significant.
DISCUSSIONUp to now, a clinical evaluation of AD using two scoring systems in a selected population of young children and adolescents in Brazil has not been reported. Since the review by Schmitt et al in 2007, the SCORAD and EASI from 20 different outcome measurements have been adequately validated.
This study shows excellent agreement between observers in the assessment of the overall AD severity using the EASI score. However, more variation between observers in assessing the severity of AD was observed in the SCORAD. Similar results were observed previously by Spriekkelman et al14.
In our study, two investigators have been chosen for scoring AD to evalúate inter-observer variation reports for extent and intensity items. Each one of the two main items of the SCORAD index (extent and subjective symptoms) was positively correlated to each other in our patients. There was not a positive correlation to each other for Intensity. However, significant variation between baseline evaluation and last evaluation was found in most severity assessment items for the SCORAD, except for Erythema.
However, significant variation between both evaluations was found in most severity assessment items for the EASI. There was no significant variation when we analysed Isolated Extent (upper limbs) (disease extent and percent of body surface area involved) for the EASI. This suggests that these scoring systems are not suitable for follow-up of the Isolated Extent item (upper limbs).
The utility of EASI in detecting changes in disease severity over the course of treatment, and its potential effectiveness in differentiating between treatment potencies has now been assessed in several clinical trial settings15–19.
Another conclusion from our data is that there was a good agreement between the two scoring systems in the assessment of the overall severity of AD. It is possible to compare results of studies on the overall severity or follow-up data of AD when these different scoring systems have been used.
Previously, it has been shown that measurements of body surface area (BSA) involvement in atopic eczema do not correlate well, representing very poor inter-observer agreement20. In contrast, we have observed a significant positive correlation between BSA assessment of SCORAD and EASI.
The EASI score is more elaborate than the SCORAD. In the former scoring system, the intensity is analysed for each body region and depends on the patient's age. On the other hand, the SCORAD index takes into account the extent of AD lesions, the lesions' intensity, and subjective symptoms, pruritus, and sleep disturbance. In the SCORAD each item is evaluated at its average intensity. Subjective symptoms represent 20 % of total SCORAD and are extremely variable (submitted to several situations, environmental exposure).
It appears that each scoring system has its own application in clinical practice and research. When a rough estimate of the severity of AD is needed to evaluate the development of allergic symptoms, a simple and rapid scoring system such as the SCORAD, assessed by a single observer, may be sufficient for this purpose. However, for detection of subtle modifications in the severity of AD, as in drugeffect studies, or for follow-up of the progression of AD for research purposes, a more elaborate scoring system, such as the EASI would be more appropriate to use.
In conclusion, both scoring systems for AD are valid, reproducible and responsive in monitoring Brazilian AD patients. The SCORAD index showed itself to be an excellent score to detect the development of AD, whereas the EASI is suitable to follow up in drug-effect studies of AD for research purposes.