The association regarding the atopic sensitization to mite aeroallergens and the socio-environmental features is still inconsistent.
ObjectivesWe analyzed the role played by socioeconomic and environmental factors in the prevalence of sensitization to house dust mite (HDM) allergens, and associated with the risk of developing asthma symptoms.
Patients and methodsThis is a case–control study conducted with 108 patients, aged 1–17. We inquired about family habits, socioeconomic and environmental features. We applied the International Study of Asthma and Allergies in Childhood questionnaire.
ResultsWe observed patients sensitized to all HDM tested, Derp (42%), Derf (37%) and Blot (33%). Middle family income (OR: 2.74; CI95%: 1.127–6.684), exposure to dog (OR: 3.758, CI95%: 1.127–6.684) and artificial climatization (OR: 4.319, CI95%: 1.398–13.348) were associated with sensitization to Derp. We also observed protective factors, such as sharing of dormitories, washing cycle for bedspreads and the presence of basic sanitation. An increased risk of sensitization to Derf was associated with Blot sensitization (OR: 3.172, CI95%: 1.083–9.292) and presence of mold on the walls (OR: 3.095, CI95%: 1.063–9.008). A protective factor was dormitory sharing. For sensitization to Blot, we observed an increase in the risk associated with Derp sensitization (OR: 3.462, CI95%: 1.191–10.061) and exposure to dog (OR: 3.255, CI95%: 0.987–10.736). In addition, sensitization to Blot increases the risk of developing asthma symptoms (OR: 2.732, CI95%: 0.981–7.606).
ConclusionOur data show distinct sociodemographic and environmental relations that lead to HDM sensitization and increased probability of development of allergic diseases.
Allergic diseases, such as asthma, rhinitis and atopic dermatitis, affect 10–30% of the worldwide population.1,2 These atopic diseases, which mainly affect children,3 are characterized by the immediate hypersensitivity mechanism, generally triggered by environmental allergens.4 Mites are considered the main aeroallergens involved in atopic manifestations.5 They belong to the order of arthropods, Arachnida class, being taxonomically related to ticks, spiders, and scorpions. Mites have a cosmopolitan distribution and are abundant in household dust, mainly in curtains, carpets, and mattresses; and their main source of food are scales of human skin (keratin), cellulose fibers and chitin.6
Dermatophagoides pteronyssinus (Derp), Dermatophagoides farinae (Derf) and Blomia tropicalis (Blot) are the main household dust mites (HDM) involved in allergic reactions. HDM produce potent allergens, such as Der p1, Der p2, Der p3 and Der p7, Der f1 and Der f2, and Blot 5.7–9 These antigens are expressed by cells from the intestinal tract, and are composed of glycoproteins and cysteine proteases.10
Geographic distribution of mites depends on temperature, humidity, and even urbanization degree.11 Ideal conditions for HDM proliferation are temperatures between 20 and 25°C associated with elevated humidity (70–80%).12 The Derp is present worldwide.12 Moreover, Derp and Derf are present in Pakistan, in Mediterranean regions of Spain,13,14 while Blot appears more frequently in tropical and subtropical regions.15
Thus, those climatic conditions and HDM distribution may affect the pattern for human sensitization to mite allergens. In addition to this, demographic density, socioeconomic status and type of housing can lead to a high prevalence of HDM sensitization and development of atopy. These conditions mainly influence the first year of life, concerning the development of allergic disorders.16
In the context, this study aims to evaluate how children and adolescents’ sensitization to Derp, Derf and Blot can change according to socioeconomic and environmental variables, and how this could affect the risk for asthma.
Material and methodsPatientsA case–control study carried out between 2017 and 2018 in the outpatient clinic from Hospital da Criança, in the city of São Luís, Maranhão, Northeast Brazil. It is a public hospital that provides specialized care for children and adolescents from different cities of the state. It is a hospital that receives people who participate in the unique health program (SUS) offered by the government. These patients were selected through medical records, and included boys and girls, aged between one and seventeen years, with or without allergic diseases. The main non-inclusion criterion was the existence of other respiratory diseases, such as Chronic Obstructive Pulmonary Disease and Cystic Fibrosis.
This study was approved by the Ethics Committee on Human Research at CEUMA University (58737916.3.0000.5084). All patients and guardians, along with their child, were informed about the research and signed written consent in order to participate. Furthermore, this study received support from Fundação de Amparo à Pesquisa do Estado do Maranhão (UNIVERSAL-01516/16).
QuestionnairesThe International Study of Asthma and Allergies in Childhood (ISAAC)17 questionnaire was applied to parents to investigate the symptoms of asthma, wheezing, rhinitis, and atopic eczema. In addition to this, a socio-environmental structured inquiry was applied, which included questions regarding: family income; level of mothers’ education; breastfeeding; family history of allergies (brothers, sisters, parents); dormitory conditions (shared, air conditioner in room, presence of mold on walls, cycle of washing quilts per week, frequent change of mattress and pillow); nursery experience in early childhood; exposure to dogs, cats and/or farm animals; and basic sanitation.
Skin prick test (prick test)Skin prick test was performed using extracts from house dust mites (Derp, Derf, Blot), cat, dog, grass, egg, and milk; buffered saline solution as a negative control; and histamine as positive control was used (Immunotech-FDA Allergenic LTDA). The test was performed at the anterior face of the forearm, as previously described and following the manufacturer's guidelines.18 After thirty minutes the reaction was evaluated. Papule with a diameter equal or greater than 3mm is considered as a positive reaction.
Statistical analysisStatistical analysis was performed using Prism 8.0 (GraphPad Software, CA, USA). For clinical and environmental data analysis, 2×2 type contingency tables were created based on chi-square and Fisher's test. In order to identify associations between variables, the Binary Logistic Regression method was used in the STATplus software (Analystsoft, CA, USA). For both analyses, Odd Ratios (OR) and the 95% confidence interval were calculated. Statistical significance was defined by p≤0.05 value.
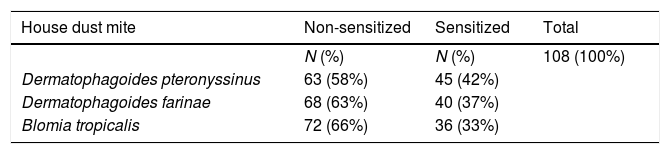
ResultsOur study sample included 108 children and adolescents, from whom 45 were sensitized to Derp (42%), 40 to Derf (37%) and 36 to Blot (33%), Table 1.
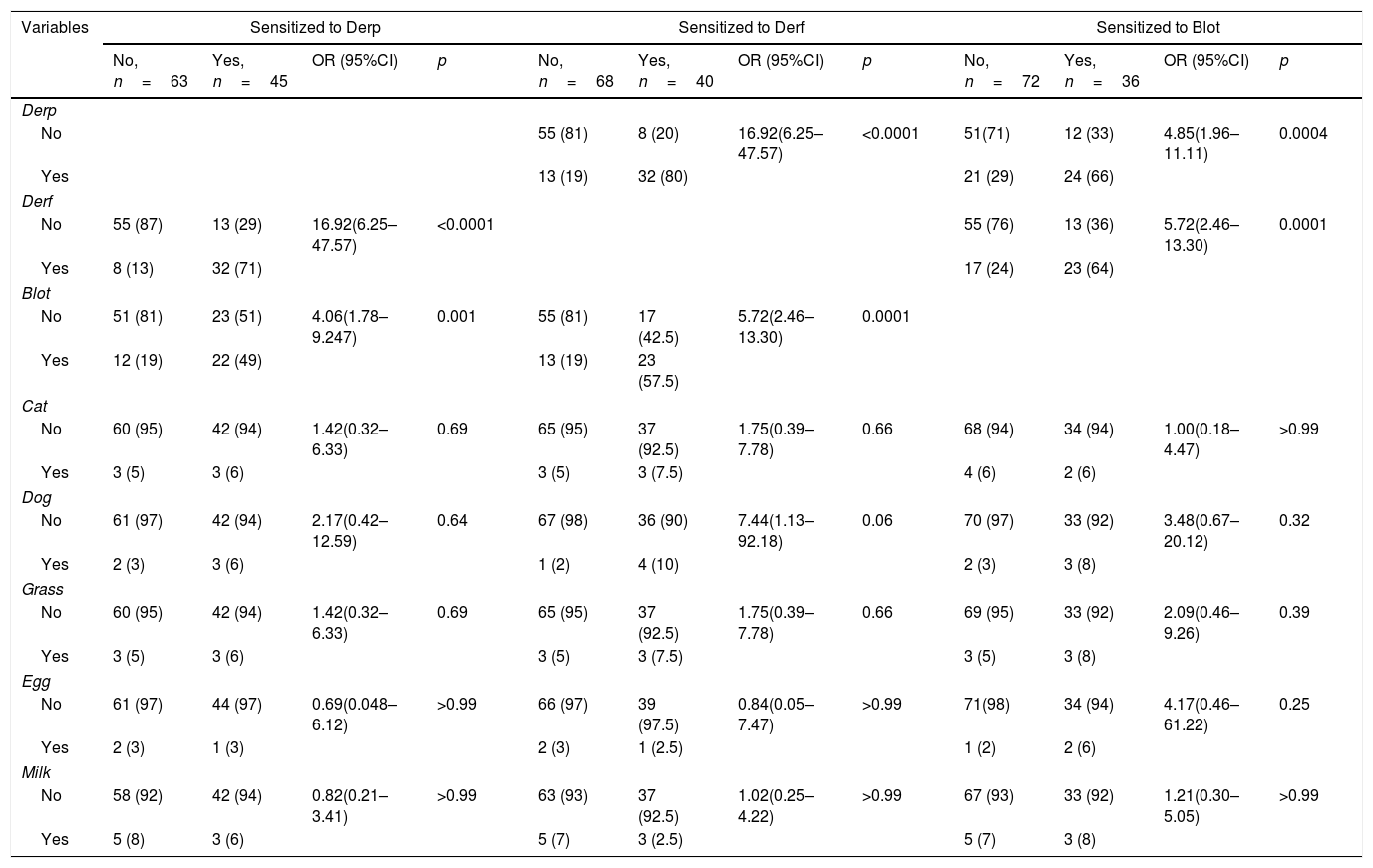
Derp, Derf, and Blot were analyzed separately in the Derp-sensitized patients. In patients sensitized to the Derp, 71% were also sensitized to Derf (p<0.0001, OR: 16.92, CI95%: 6.25–47.57) and 49% were sensitized to Blot (p=0.0015, OR: 4.06, CI95%: 1.78–9.24) (Table 2).
Sensitization to HDM and to allergens from other sources.
| Variables | Sensitized to Derp | Sensitized to Derf | Sensitized to Blot | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No, n=63 | Yes, n=45 | OR (95%CI) | p | No, n=68 | Yes, n=40 | OR (95%CI) | p | No, n=72 | Yes, n=36 | OR (95%CI) | p | |
| Derp | ||||||||||||
| No | 55 (81) | 8 (20) | 16.92(6.25–47.57) | <0.0001 | 51(71) | 12 (33) | 4.85(1.96–11.11) | 0.0004 | ||||
| Yes | 13 (19) | 32 (80) | 21 (29) | 24 (66) | ||||||||
| Derf | ||||||||||||
| No | 55 (87) | 13 (29) | 16.92(6.25–47.57) | <0.0001 | 55 (76) | 13 (36) | 5.72(2.46–13.30) | 0.0001 | ||||
| Yes | 8 (13) | 32 (71) | 17 (24) | 23 (64) | ||||||||
| Blot | ||||||||||||
| No | 51 (81) | 23 (51) | 4.06(1.78–9.247) | 0.001 | 55 (81) | 17 (42.5) | 5.72(2.46–13.30) | 0.0001 | ||||
| Yes | 12 (19) | 22 (49) | 13 (19) | 23 (57.5) | ||||||||
| Cat | ||||||||||||
| No | 60 (95) | 42 (94) | 1.42(0.32–6.33) | 0.69 | 65 (95) | 37 (92.5) | 1.75(0.39–7.78) | 0.66 | 68 (94) | 34 (94) | 1.00(0.18–4.47) | >0.99 |
| Yes | 3 (5) | 3 (6) | 3 (5) | 3 (7.5) | 4 (6) | 2 (6) | ||||||
| Dog | ||||||||||||
| No | 61 (97) | 42 (94) | 2.17(0.42–12.59) | 0.64 | 67 (98) | 36 (90) | 7.44(1.13–92.18) | 0.06 | 70 (97) | 33 (92) | 3.48(0.67–20.12) | 0.32 |
| Yes | 2 (3) | 3 (6) | 1 (2) | 4 (10) | 2 (3) | 3 (8) | ||||||
| Grass | ||||||||||||
| No | 60 (95) | 42 (94) | 1.42(0.32–6.33) | 0.69 | 65 (95) | 37 (92.5) | 1.75(0.39–7.78) | 0.66 | 69 (95) | 33 (92) | 2.09(0.46–9.26) | 0.39 |
| Yes | 3 (5) | 3 (6) | 3 (5) | 3 (7.5) | 3 (5) | 3 (8) | ||||||
| Egg | ||||||||||||
| No | 61 (97) | 44 (97) | 0.69(0.048–6.12) | >0.99 | 66 (97) | 39 (97.5) | 0.84(0.05–7.47) | >0.99 | 71(98) | 34 (94) | 4.17(0.46–61.22) | 0.25 |
| Yes | 2 (3) | 1 (3) | 2 (3) | 1 (2.5) | 1 (2) | 2 (6) | ||||||
| Milk | ||||||||||||
| No | 58 (92) | 42 (94) | 0.82(0.21–3.41) | >0.99 | 63 (93) | 37 (92.5) | 1.02(0.25–4.22) | >0.99 | 67 (93) | 33 (92) | 1.21(0.30–5.05) | >0.99 |
| Yes | 5 (8) | 3 (6) | 5 (7) | 3 (2.5) | 5 (7) | 3 (8) | ||||||
Values in parentheses represent %.
Chi-square and Fisher's test. OR: odd ratios; CI: confidence interval.
In patients sensitized to the Derf, 80% were also sensitized to Derp (p<0.0001, OR: 16.92, CI95%: 6.25–47.57) and 57.5% to Blot (p=0.0001, OR: 5.72, CI95%: 2.46–13.30) (Table 2).
In patients sensitized to Blot, 66% were also sensitized to Derp (p=0.0004, OR: 4.85, CI95%: 1.96–11.11) and 64% to Derf (p=0.0001, OR: 5.75 CI95%: 2.46–13.3) (Table 2).
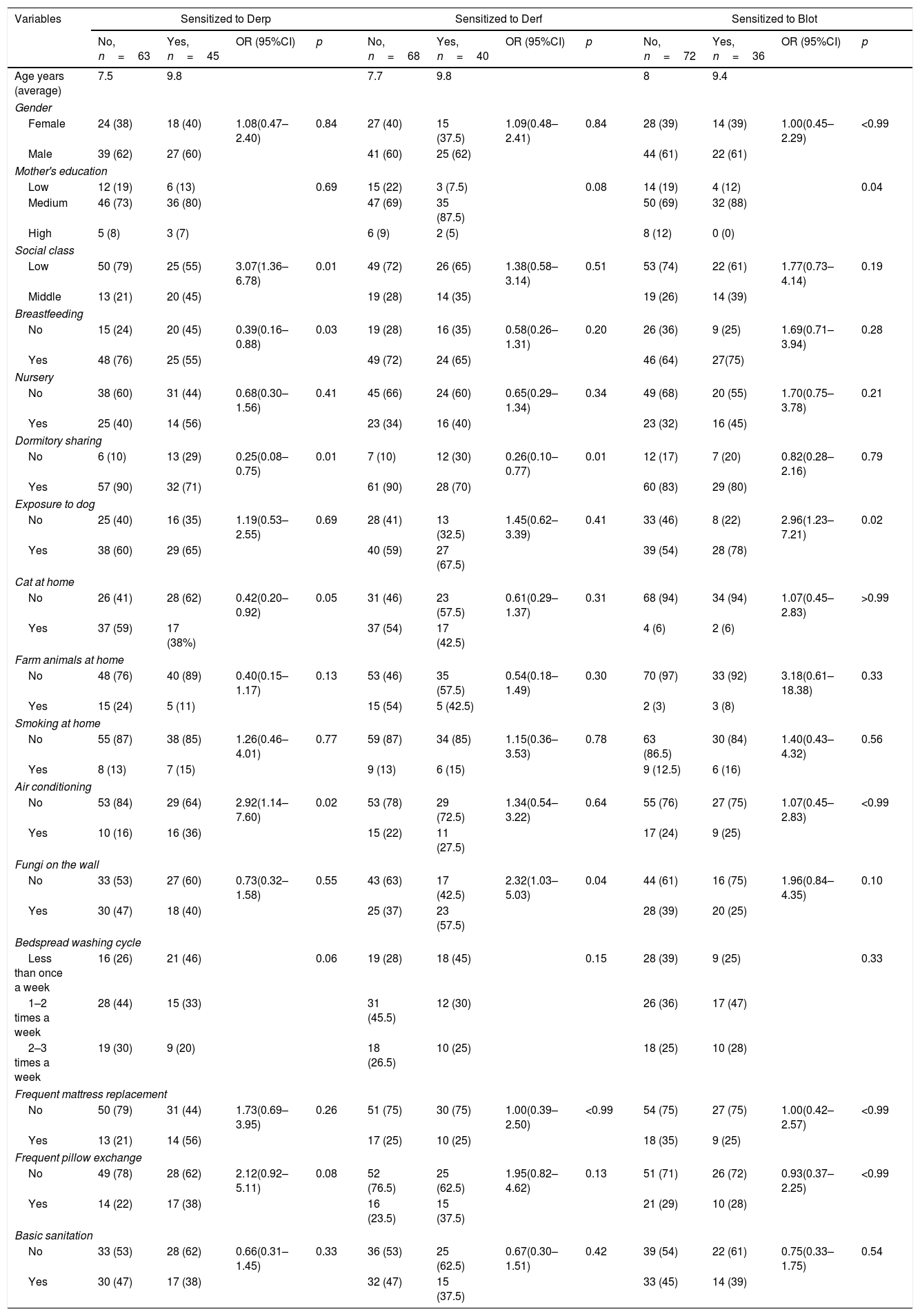
Socio-environmental analyses showed that social class (p=0.01, OR: 3.077, CI95%: 1.362–6.785) and room with air-conditioner (p=0.02, OR: 2.924, CI95%: 1.144–7.607) are risk factors for Derp sensitization (Table 3). On the other hand, breastfeeding in the first months of life (55%; p=0.03, OR: 0.390, CI: 0.169–0.884) and share dormitory (71%; p=0.03; OR: 0.259, CI95%: 0.088–0.757) were shown as protective factors against Derp sensitization (Table 3).
Patients’ socio-environmental profile.
| Variables | Sensitized to Derp | Sensitized to Derf | Sensitized to Blot | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No, n=63 | Yes, n=45 | OR (95%CI) | p | No, n=68 | Yes, n=40 | OR (95%CI) | p | No, n=72 | Yes, n=36 | OR (95%CI) | p | |
| Age years (average) | 7.5 | 9.8 | 7.7 | 9.8 | 8 | 9.4 | ||||||
| Gender | ||||||||||||
| Female | 24 (38) | 18 (40) | 1.08(0.47–2.40) | 0.84 | 27 (40) | 15 (37.5) | 1.09(0.48–2.41) | 0.84 | 28 (39) | 14 (39) | 1.00(0.45–2.29) | <0.99 |
| Male | 39 (62) | 27 (60) | 41 (60) | 25 (62) | 44 (61) | 22 (61) | ||||||
| Mother's education | ||||||||||||
| Low | 12 (19) | 6 (13) | 0.69 | 15 (22) | 3 (7.5) | 0.08 | 14 (19) | 4 (12) | 0.04 | |||
| Medium | 46 (73) | 36 (80) | 47 (69) | 35 (87.5) | 50 (69) | 32 (88) | ||||||
| High | 5 (8) | 3 (7) | 6 (9) | 2 (5) | 8 (12) | 0 (0) | ||||||
| Social class | ||||||||||||
| Low | 50 (79) | 25 (55) | 3.07(1.36–6.78) | 0.01 | 49 (72) | 26 (65) | 1.38(0.58–3.14) | 0.51 | 53 (74) | 22 (61) | 1.77(0.73–4.14) | 0.19 |
| Middle | 13 (21) | 20 (45) | 19 (28) | 14 (35) | 19 (26) | 14 (39) | ||||||
| Breastfeeding | ||||||||||||
| No | 15 (24) | 20 (45) | 0.39(0.16–0.88) | 0.03 | 19 (28) | 16 (35) | 0.58(0.26–1.31) | 0.20 | 26 (36) | 9 (25) | 1.69(0.71–3.94) | 0.28 |
| Yes | 48 (76) | 25 (55) | 49 (72) | 24 (65) | 46 (64) | 27(75) | ||||||
| Nursery | ||||||||||||
| No | 38 (60) | 31 (44) | 0.68(0.30–1.56) | 0.41 | 45 (66) | 24 (60) | 0.65(0.29–1.34) | 0.34 | 49 (68) | 20 (55) | 1.70(0.75–3.78) | 0.21 |
| Yes | 25 (40) | 14 (56) | 23 (34) | 16 (40) | 23 (32) | 16 (45) | ||||||
| Dormitory sharing | ||||||||||||
| No | 6 (10) | 13 (29) | 0.25(0.08–0.75) | 0.01 | 7 (10) | 12 (30) | 0.26(0.10–0.77) | 0.01 | 12 (17) | 7 (20) | 0.82(0.28–2.16) | 0.79 |
| Yes | 57 (90) | 32 (71) | 61 (90) | 28 (70) | 60 (83) | 29 (80) | ||||||
| Exposure to dog | ||||||||||||
| No | 25 (40) | 16 (35) | 1.19(0.53–2.55) | 0.69 | 28 (41) | 13 (32.5) | 1.45(0.62–3.39) | 0.41 | 33 (46) | 8 (22) | 2.96(1.23–7.21) | 0.02 |
| Yes | 38 (60) | 29 (65) | 40 (59) | 27 (67.5) | 39 (54) | 28 (78) | ||||||
| Cat at home | ||||||||||||
| No | 26 (41) | 28 (62) | 0.42(0.20–0.92) | 0.05 | 31 (46) | 23 (57.5) | 0.61(0.29–1.37) | 0.31 | 68 (94) | 34 (94) | 1.07(0.45–2.83) | >0.99 |
| Yes | 37 (59) | 17 (38%) | 37 (54) | 17 (42.5) | 4 (6) | 2 (6) | ||||||
| Farm animals at home | ||||||||||||
| No | 48 (76) | 40 (89) | 0.40(0.15–1.17) | 0.13 | 53 (46) | 35 (57.5) | 0.54(0.18–1.49) | 0.30 | 70 (97) | 33 (92) | 3.18(0.61–18.38) | 0.33 |
| Yes | 15 (24) | 5 (11) | 15 (54) | 5 (42.5) | 2 (3) | 3 (8) | ||||||
| Smoking at home | ||||||||||||
| No | 55 (87) | 38 (85) | 1.26(0.46–4.01) | 0.77 | 59 (87) | 34 (85) | 1.15(0.36–3.53) | 0.78 | 63 (86.5) | 30 (84) | 1.40(0.43–4.32) | 0.56 |
| Yes | 8 (13) | 7 (15) | 9 (13) | 6 (15) | 9 (12.5) | 6 (16) | ||||||
| Air conditioning | ||||||||||||
| No | 53 (84) | 29 (64) | 2.92(1.14–7.60) | 0.02 | 53 (78) | 29 (72.5) | 1.34(0.54–3.22) | 0.64 | 55 (76) | 27 (75) | 1.07(0.45–2.83) | <0.99 |
| Yes | 10 (16) | 16 (36) | 15 (22) | 11 (27.5) | 17 (24) | 9 (25) | ||||||
| Fungi on the wall | ||||||||||||
| No | 33 (53) | 27 (60) | 0.73(0.32–1.58) | 0.55 | 43 (63) | 17 (42.5) | 2.32(1.03–5.03) | 0.04 | 44 (61) | 16 (75) | 1.96(0.84–4.35) | 0.10 |
| Yes | 30 (47) | 18 (40) | 25 (37) | 23 (57.5) | 28 (39) | 20 (25) | ||||||
| Bedspread washing cycle | ||||||||||||
| Less than once a week | 16 (26) | 21 (46) | 0.06 | 19 (28) | 18 (45) | 0.15 | 28 (39) | 9 (25) | 0.33 | |||
| 1–2 times a week | 28 (44) | 15 (33) | 31 (45.5) | 12 (30) | 26 (36) | 17 (47) | ||||||
| 2–3 times a week | 19 (30) | 9 (20) | 18 (26.5) | 10 (25) | 18 (25) | 10 (28) | ||||||
| Frequent mattress replacement | ||||||||||||
| No | 50 (79) | 31 (44) | 1.73(0.69–3.95) | 0.26 | 51 (75) | 30 (75) | 1.00(0.39–2.50) | <0.99 | 54 (75) | 27 (75) | 1.00(0.42–2.57) | <0.99 |
| Yes | 13 (21) | 14 (56) | 17 (25) | 10 (25) | 18 (35) | 9 (25) | ||||||
| Frequent pillow exchange | ||||||||||||
| No | 49 (78) | 28 (62) | 2.12(0.92–5.11) | 0.08 | 52 (76.5) | 25 (62.5) | 1.95(0.82–4.62) | 0.13 | 51 (71) | 26 (72) | 0.93(0.37–2.25) | <0.99 |
| Yes | 14 (22) | 17 (38) | 16 (23.5) | 15 (37.5) | 21 (29) | 10 (28) | ||||||
| Basic sanitation | ||||||||||||
| No | 33 (53) | 28 (62) | 0.66(0.31–1.45) | 0.33 | 36 (53) | 25 (62.5) | 0.67(0.30–1.51) | 0.42 | 39 (54) | 22 (61) | 0.75(0.33–1.75) | 0.54 |
| Yes | 30 (47) | 17 (38) | 32 (47) | 15 (37.5) | 33 (45) | 14 (39) | ||||||
Values in parentheses represent %.
Chi-square and Fisher's test. OR: odds ratio; CI: confidence interval.
Regarding sensitization to Derf, dormitory sharing (70%, p=0.01, OR: 0.267, CI95%: 0.104–0.777) was a protective factor, while presence of mold (fungi) on the wall (57.5%, p=2.327, OR=0.04, CI95%: 1.035–5.034) was a risk factor (Table 3).
For patients sensitized to Blot, mothers’ level of education (88% with a medium level of education; p=0.04) presented as a protective factor. However, exposure to dog was showed as a risk factor (78%, p=0.02, OR: 2.962, CI95%: 1.232–7.216) (Table 3).
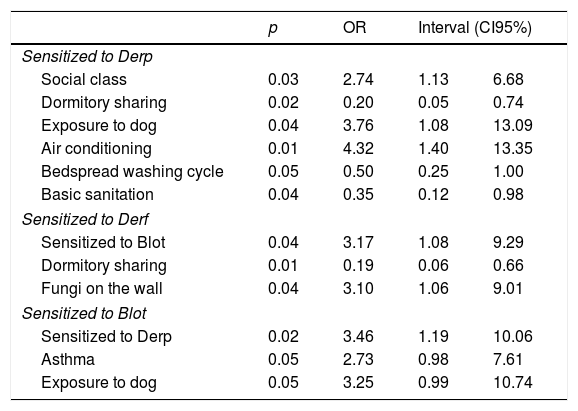
In logistic regression analyses, it was observed that the increased risk of sensitization to Derp was associated to: middle social class (p=0.02, OR: 2.74, CI95%: 1.12–6.68); frequent exposure to dog (p=0.03, OR: 3.75, CI95%: 1.12–6.68); artificial climatization of the room (p=0.01, OR: 4.31, CI95%: 1.39–13.34). Protective factors against sensitization to Derp were associated to: dormitory sharing (p=0.01, OR: 0.20, CI95%: 0.055–0.74); frequent washing of bedding (p=0.05, OR: 0.49, CI95%: 0.25–0.99); and housing with basic sanitation (p=0.01; OR: 4.31; CI95%: 1.39–13.34) (Table 4).
Analyses of the regression binary logistics of variables.
| p | OR | Interval (CI95%) | ||
|---|---|---|---|---|
| Sensitized to Derp | ||||
| Social class | 0.03 | 2.74 | 1.13 | 6.68 |
| Dormitory sharing | 0.02 | 0.20 | 0.05 | 0.74 |
| Exposure to dog | 0.04 | 3.76 | 1.08 | 13.09 |
| Air conditioning | 0.01 | 4.32 | 1.40 | 13.35 |
| Bedspread washing cycle | 0.05 | 0.50 | 0.25 | 1.00 |
| Basic sanitation | 0.04 | 0.35 | 0.12 | 0.98 |
| Sensitized to Derf | ||||
| Sensitized to Blot | 0.04 | 3.17 | 1.08 | 9.29 |
| Dormitory sharing | 0.01 | 0.19 | 0.06 | 0.66 |
| Fungi on the wall | 0.04 | 3.10 | 1.06 | 9.01 |
| Sensitized to Blot | ||||
| Sensitized to Derp | 0.02 | 3.46 | 1.19 | 10.06 |
| Asthma | 0.05 | 2.73 | 0.98 | 7.61 |
| Exposure to dog | 0.05 | 3.25 | 0.99 | 10.74 |
Increased risk of sensitization to Derf was associated with Blot sensitization (p=0.03; OR: 3.17; CI95%: 1.08–9.29) and the presence of mold (fungi) on the walls (p=0.03, OR: 3.095, CI95%: 1.06–9.00). However, dormitory sharing was associated as a protective factor to Derf (p=0.009, OR: 0.19, CI95%: 0.05–0.65) (Table 4).
Increased risk of sensitization to Blot were associated with Derp sensitization (p=0.02, OR: 3.462, CI95%: 1.191–10.061) and exposure to dog (p=0.05, OR: 3.255, CI95%: 0.987–10.736). In addition, sensitization to Blot increases risk of developing asthma symptoms (p=0.05, OR: 2.732, CI95%: 0.981–7.606) (Table 4).
DiscussionThe relation of humankind with the surrounding environment, in addition to socioeconomic conditions, constitutes fundamental factors in the development of different pathologies. In the case of atopy, it is quite evident that exposure to house dust mites (HDM) is a risk factor for the development of wheezing, asthma, among others.19 In this study, we demonstrated that sociodemographic elements, such as social class, mothers’ education and environment factors, such as exposure to dog, presence of mold on the wall and air conditioner, are associated with mite allergen sensitization. This data provides pieces of evidence about the relevance of those factors in allergy scenario.
Respiratory atopic reactions are frequently associated with sensitization to HDM allergens.11 It is estimated that 1–2% of the world population has allergic sensitization to HDM, with significant variability depending on the cohort studied.20 In our study population, it was possible to identify the sensitization by the three main mite allergens, Derp (42%), Derf (37%) and Blot (33%). This high percentage of HDM sensitization, compared to the world prevalence, could probably be related to weather conditions for mite proliferation.
The northeast region of Brazil is characterized by a tropical climate, mainly by two well defined, rainy and dry, seasons, with temperatures between 25°C and 27°C. The city of São Luís, Maranhão, presents characteristics of a tropical climate, with high temperatures and humidity, showing favorable environmental conditions for mite development. Besides, because it is a coastal city, maritime influences can enhance the proliferation of the acarine population. The effect of moisture can be observed in British Columbia, Canada, which presents characteristics of high humidity associated to the high proliferation of HDM.12 This prevalence of mites is closely related to the significant prevalence of HDM sensitization.21
Sensitization to HDM is an important parameter to understand the epidemiology of atopies. Our results show a statistical association with clinical symptoms of asthma and sensitization to Blot. In 1997, Arruda et al. reported sensitization to Blot in asthmatic patients, and also the identification of Blot 5 allergen.22 These are fundamental data to understand the triggers of allergic diseases in our study population.
However, while allergic sensitization is known to increase the risk for allergic diseases, there is relevant heterogeneity among allergen-sensitized children and sometimes an absence of atopy development. The association between different allergens increases the risk of developing allergic diseases.23 Moreover, we did not observe associations between sensitization to HDM allergens and sensitization to food or atopic dermatitis-related allergens. This heterogeneous response to sensitization could explain why we did not find a statistically significant association between sensitization to HDM with other allergic symptoms, such as rhinitis, eczema, and wheezing (data not shown).
Other factors that help us to further understand the prevalence of aeroallergen sensitization are socioeconomic conditions. Our work shows that family income and the level of education of the mother are involved in sensitization to HDM. Even more, patients that belong to low- and middle-income families present an increased risk to become sensitized to HDM. Additionally, our data shows a higher prevalence of medium level of education of the mother is associated in sensitization to HDM. Related to that, some countries such as the United States, Canada, the United Kingdom, Japan, India, Brazil show an association between low socioeconomic status and low lung function in childhood.16,24,25
The mother–child relationship, since birth, is another fundamental element in infant health and development. In our study, it was observed that breastfeeding is a protective factor versus sensibilization to Derp. Several studies have demonstrated that breastfeeding reduces the risk of developing asthma.26 However, in 2017 one research group proposed that oral exposure to aeroallergens through breast milk may affect the risk of developing allergies. They demonstrated that the presence of Derp and Blot in human breast milk is associated with respiratory allergy. A mouse model showed that Derp in breast milk promoted allergic sensitization in the progeny.27 Thus, we need further evaluations to better understand the mother–child relationship in our cohort.
Regarding housing conditions, as well as the family habits, some studies showed that they are also determinant risk factors for mite proliferation, sensitization to aeroallergens and triggering of atopy, such as asthma.28 Our results show that factors such as air conditioner, presence of mold, and exposure to dog are determinant for sensitization to HDM. These have already been described as triggers for allergic diseases, especially in the first years of life.29 The presence of domesticated animals, for example, is related to the increase of HDM proliferation in family environments.30
In the last few years, environmental features have been shown to be as important as the genetic heritage to determine the development of allergies. The investigation of the prevalence of sensitization to HDM allergens, specifically Derp, Derf, and Blot, together with the identification of the main atopic manifestations, evaluation of socioeconomic and environmental factors, allow us a more specific understanding of the interaction between humankind and the environment that leads to atopy conditions.
ConclusionIn conclusion, we found that socio-behavioral and economic factors, such as social class, maternal education, constant dog exposure, presence of wall fungus and air conditioner were risk factors in the sensitization to HDM. On the other hand, elements such as dormitory sharing, breastfeeding and basic sanitation contribute as a protective factor against sensitization to HDM. Besides, patients sensitized for Blot were associated with asthma symptoms.
The high prevalence of sensitized patients to HDM allergens suggests exposure to high concentrations of these allergens or, at least, to constant exposure to those mites. Further studies to associate HDM concentrations and sociodemographic data are required. However, our data can suggest a new insight for physicians in understanding important factors that define allergic patients’ manifestations, providing an improvement in their quality of life.
FundingThe funding agencies supported this work: the Foundation for Research and Scientific and Technological Development of Maranhão – FAPEMA (UNIVERSAL-01516/16).
Conflict of interestThe authors have no conflict of interest to declare.
As authors of this study, we wish to express our gratitude to the parents and children that kindly chose to partake in our Project; as well as the health professionals at the Children's Hospital who assist us in the recruitment of patients.
We would also like to thank all professors, undergraduates and graduate students frequenting the environmental microbiology laboratory.