The present cross-sectional study investigated the associations between low birthweight (LBW), high birthweight, preterm birth (PTB), postterm birth, small for gestational age (SGA), and large for gestational age (LGA) and the prevalence of wheeze and asthma in Japanese children aged three years (age range, 33–54 months; mean age, 38.7 months).
MethodsStudy subjects were 6364 children. A questionnaire was used to collect all data. Wheeze and asthma were defined according to the criteria of the International Study of Asthma and Allergies in Childhood.
ResultsThe prevalence values of wheeze and asthma were 19.5% and 7.7%, respectively. Of the 6364 subjects, 8.8% were classified as LBW (<2500g), 90.4% as normal birthweight, 0.8% as high birthweight (≥4000g), 4.8% as PTB (<37 weeks), 94.8% as term birth, 0.4% as postterm birth (≥42 weeks), 7.8% as SGA (<10th percentile), 82.5% as appropriate for gestational age, and 9.7% as LGA (>90th percentile). Compared with term birth, PTB was independently positively associated with wheeze and asthma: the adjusted ORs (95% CI) were 1.47 (1.11–1.92) and 1.52 (1.02–2.20), respectively. An independent positive association was shown between PTB and wheeze only in boys; the interaction between PTB and sex was significant. Such an interaction between PTB and sex was not seen for asthma. No evident associations were observed between LBW, high birthweight, postterm birth, SGA, or LGA and wheeze or asthma.
ConclusionsThis is the first study in Japan to show that PTB, but not LBW or SGA, was significantly positively associated with childhood wheeze and asthma.
Asthma and wheeze remain common public health problems in childhood. There is growing evidence that birth characteristics may affect asthma and wheeze, based on epidemiological studies conducted mainly in Western countries.1–5 In 2015, a meta-analysis of 37 studies comprising 1,712,737 children showed a significant positive association between low birthweight (LBW) (<2.5kg) and wheezing disorders while high birthweight (>4.0kg) was not significantly related to wheezing disorders.4 A meta-analysis for 147,252 European children in 31 birth cohort studies demonstrated that both LBW and preterm birth (PTB) were significantly positively related to preschool wheezing and school-age asthma.5 Another meta-analysis in 2014 of 30 studies including 1,543,639 children found a significant positive association between PTB and wheezing disorders.1 Epidemiological evidence on the relationship between small for gestational age (SGA) and childhood asthma is limited2,3,6,7; to our knowledge, a meta-analysis has not been conducted.
In Japan, only one cross-sectional study has examined this issue, showing no associations between LBW, PTB, or SGA and wheeze or asthma in 2004 children aged three years.8 In the present study, we cross-sectionally investigated the effect of not only LBW, PTB, and SGA but also high birthweight, postterm birth, and large for gestational age (LGA) on wheeze and asthma in Japanese children aged three years using data from the Kyushu Okinawa Child Health Study (KOCHS).
Materials and methodsStudy subjectsIn Japan, under the Maternal and Child Health Law, each municipality provides physical examinations for its resident children when they reach the age of three. Eligible study subjects were 68,527 children aged three years who lived in one of 32 municipalities; these were Fukuoka City, a metropolitan area in Fukuoka Prefecture, six municipalities in five prefectures other than Fukuoka Prefecture on Kyushu Island in southern Japan and seven municipalities in Okinawa Prefecture, an island chain in the southwest of Japan. Each subject received a physical examination at a public health center in his or her municipality of residence between May 2012 and March 2014. At the time of the physical examination, we aimed to provide all 68,527 eligible children's parents or guardians with a 38-page self-administered questionnaire and a postage-paid, addressed return envelope; in practice, however, the parents or guardians of 62,449 children actually took the materials home with them. In the end, a total of 6576 parents or guardians gave their written informed consent and mailed the completed questionnaire to the data management center (participation rate=9.6%). Our research technicians completed missing answers and/or illogical data by telephone interview with individual parents or guardians. We excluded 212 children with missing data on the variables under study. The final study group thus consisted of 6364 children (9.3% of the 68,527 eligible children). The KOCHS was approved by the ethics committees of the Faculty of Medicine, Fukuoka University and Ehime University Graduate School of Medicine.
MeasurementsIn Japan, under the Maternal and Child Health Law, each municipality provides a booklet called the Maternal and Child Health Handbook to every pregnant woman residing in the municipality at the start of her pregnancy; the Handbook is used to record data pertaining to prenatal checkups, postnatal health conditions of both mother and baby, and growth of the child. In Japan, generally, an obstetrician's estimate of gestational age at the time of delivery is based on early ultrasound examination and/or the first day of the last menstrual period, and birthweight is measured immediately after birth. Data such as birthweight and gestational age at birth are recorded by staff at the birth hospital or clinic in the Maternal and Child Health Handbook. In the KOCHS, the parents or guardians were required to transcribe the data on birthweight and gestational age at birth from the Maternal and Child Health Handbook to our self-administered questionnaire. LBW was defined as a birthweight of less than 2500g. High birthweight was defined as a birthweight of 4000g or more. Gestational age was categorized as PTB (<37 weeks), term birth (37–41 weeks), and postterm birth (≥42 weeks). SGA and LGA were defined as a birthweight below the 10th percentile and above the 90th percentile, respectively, of the Japanese neonatal anthropometric norms for babies of the same gestational age, gender, and parity published by Itabashi et al. in 20109; the remainder were considered to be appropriate for gestational age. These norms show the distribution of birthweights for each day of gestational age; in this study, however, data on gestational ages were only available in weeks. Thus, for the purposes of comparison and analysis, we selected the distributions from the third day of each week from the study of Itabashi et al.
The self-administered questionnaire included questions on wheeze and asthma, which were defined as in the International Study of Asthma and Allergies in Childhood (ISAAC).10 Wheeze was defined as present if the parent or guardian answered “yes” to the question “Has your child had wheezing or whistling in the chest in the last 12 months?” Those children who responded positively to both the abovementioned question and the following question “Has your child ever had asthma?” were considered to have asthma. The questionnaire also elicited information on maternal age at birth, date of birth, sex, region of residence, number of siblings, breastfeeding duration, maternal smoking during pregnancy, household smoking during the first year of life, paternal and maternal educational levels, household income, and paternal and maternal history of asthma, atopic eczema, and allergic rhinitis.
Statistical analysisMaternal age at birth, age, sex, region of residence, number of siblings, breastfeeding duration, maternal smoking during pregnancy, household smoking during the first year of life, paternal and maternal educational levels, household income, and paternal and maternal history of asthma, atopic eczema, and allergic rhinitis were selected as a priori potential confounding factors. Logistic regression analysis was used to estimate crude odds ratios (ORs) and their 95% confidence intervals (CIs) for wheeze and asthma in relation to birth characteristics. Multiple logistic regression analysis was performed to adjust for potential confounding factors. All computations were performed using the SAS software package version 9.4 (SAS Institute, Inc., Cary, NC, USA).
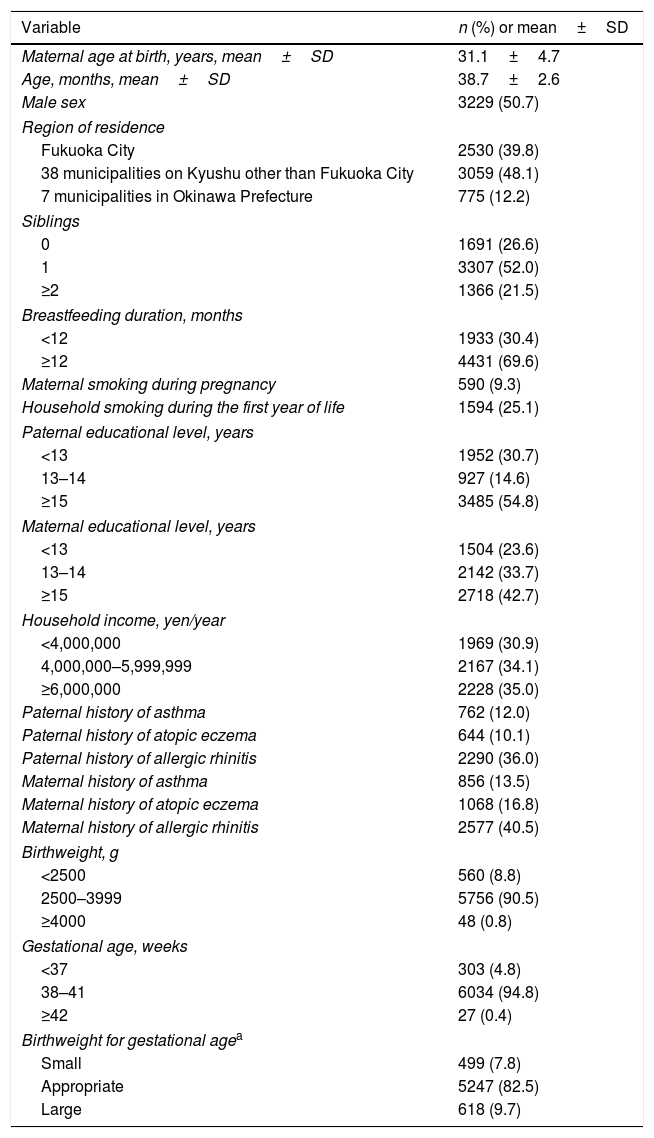
ResultsAmong the 6364 children, the prevalence values of wheeze and asthma were 19.5% and 7.7%, respectively. The mean birthweight was 3005.0g, and 8.8% were classified as LBW, 90.4% as normal birthweight, 0.8% as high birthweight, 4.8% as PTB, 94.8% as term birth, 0.4% as postterm birth, 7.8% as SGA, 82.5% in 10–90th percentile, and 9.7% as LGA (Table 1).
Distribution of selected characteristics in 6364 children aged three years.
| Variable | n (%) or mean±SD |
|---|---|
| Maternal age at birth, years, mean±SD | 31.1±4.7 |
| Age, months, mean±SD | 38.7±2.6 |
| Male sex | 3229 (50.7) |
| Region of residence | |
| Fukuoka City | 2530 (39.8) |
| 38 municipalities on Kyushu other than Fukuoka City | 3059 (48.1) |
| 7 municipalities in Okinawa Prefecture | 775 (12.2) |
| Siblings | |
| 0 | 1691 (26.6) |
| 1 | 3307 (52.0) |
| ≥2 | 1366 (21.5) |
| Breastfeeding duration, months | |
| <12 | 1933 (30.4) |
| ≥12 | 4431 (69.6) |
| Maternal smoking during pregnancy | 590 (9.3) |
| Household smoking during the first year of life | 1594 (25.1) |
| Paternal educational level, years | |
| <13 | 1952 (30.7) |
| 13–14 | 927 (14.6) |
| ≥15 | 3485 (54.8) |
| Maternal educational level, years | |
| <13 | 1504 (23.6) |
| 13–14 | 2142 (33.7) |
| ≥15 | 2718 (42.7) |
| Household income, yen/year | |
| <4,000,000 | 1969 (30.9) |
| 4,000,000–5,999,999 | 2167 (34.1) |
| ≥6,000,000 | 2228 (35.0) |
| Paternal history of asthma | 762 (12.0) |
| Paternal history of atopic eczema | 644 (10.1) |
| Paternal history of allergic rhinitis | 2290 (36.0) |
| Maternal history of asthma | 856 (13.5) |
| Maternal history of atopic eczema | 1068 (16.8) |
| Maternal history of allergic rhinitis | 2577 (40.5) |
| Birthweight, g | |
| <2500 | 560 (8.8) |
| 2500–3999 | 5756 (90.5) |
| ≥4000 | 48 (0.8) |
| Gestational age, weeks | |
| <37 | 303 (4.8) |
| 38–41 | 6034 (94.8) |
| ≥42 | 27 (0.4) |
| Birthweight for gestational agea | |
| Small | 499 (7.8) |
| Appropriate | 5247 (82.5) |
| Large | 618 (9.7) |
SD: standard deviation.
Based on the Japanese neonatal anthropometric norms for babies of the same gestational age, gender, and parity published by Itabashi et al. in 2010.9
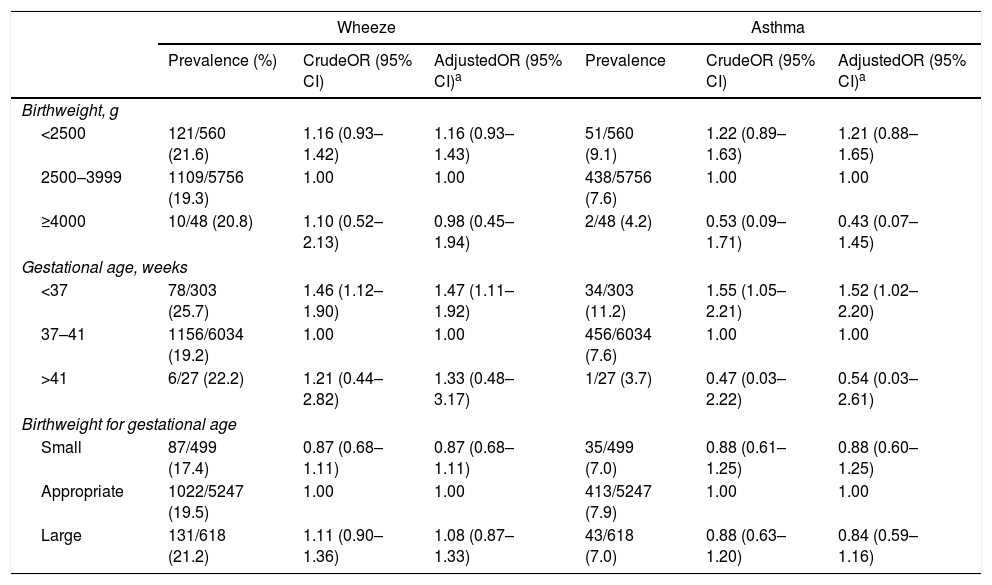
Table 2 provides ORs and 95% CIs for wheeze and asthma in relation to birth characteristics. Compared with term birth, PTB was significantly associated with a higher prevalence of wheeze while there was no relationship between postterm birth and wheeze. Adjustment for the confounding factors under study did not materially change the results: the adjusted OR for PTB was 1.47 (95% CI: 1.11–1.92). Similarly, PTB was independently positively related to the prevalence of asthma: the adjusted OR was 1.52 (95% CI: 1.02–2.20); however, no association was found between postterm birth and asthma. Compared with a birthweight of 2500–3999g, neither LBW nor high birthweight was significantly associated with wheeze or asthma in the multivariate model. Compared with appropriate size for gestational age, no significant relationships were observed between SGA or LGA and wheeze or asthma.
ORs and 95% CIs for wheeze and asthma in relation to birth characteristics in 6364 Japanese children aged three years.
| Wheeze | Asthma | |||||
|---|---|---|---|---|---|---|
| Prevalence (%) | CrudeOR (95% CI) | AdjustedOR (95% CI)a | Prevalence | CrudeOR (95% CI) | AdjustedOR (95% CI)a | |
| Birthweight, g | ||||||
| <2500 | 121/560 (21.6) | 1.16 (0.93–1.42) | 1.16 (0.93–1.43) | 51/560 (9.1) | 1.22 (0.89–1.63) | 1.21 (0.88–1.65) |
| 2500–3999 | 1109/5756 (19.3) | 1.00 | 1.00 | 438/5756 (7.6) | 1.00 | 1.00 |
| ≥4000 | 10/48 (20.8) | 1.10 (0.52–2.13) | 0.98 (0.45–1.94) | 2/48 (4.2) | 0.53 (0.09–1.71) | 0.43 (0.07–1.45) |
| Gestational age, weeks | ||||||
| <37 | 78/303 (25.7) | 1.46 (1.12–1.90) | 1.47 (1.11–1.92) | 34/303 (11.2) | 1.55 (1.05–2.21) | 1.52 (1.02–2.20) |
| 37–41 | 1156/6034 (19.2) | 1.00 | 1.00 | 456/6034 (7.6) | 1.00 | 1.00 |
| >41 | 6/27 (22.2) | 1.21 (0.44–2.82) | 1.33 (0.48–3.17) | 1/27 (3.7) | 0.47 (0.03–2.22) | 0.54 (0.03–2.61) |
| Birthweight for gestational age | ||||||
| Small | 87/499 (17.4) | 0.87 (0.68–1.11) | 0.87 (0.68–1.11) | 35/499 (7.0) | 0.88 (0.61–1.25) | 0.88 (0.60–1.25) |
| Appropriate | 1022/5247 (19.5) | 1.00 | 1.00 | 413/5247 (7.9) | 1.00 | 1.00 |
| Large | 131/618 (21.2) | 1.11 (0.90–1.36) | 1.08 (0.87–1.33) | 43/618 (7.0) | 0.88 (0.63–1.20) | 0.84 (0.59–1.16) |
CI: confidence interval; OR: odds ratio.
Adjustment for maternal age at birth, age, sex, region of residence, number of siblings, breastfeeding duration, maternal smoking during pregnancy, household smoking during the first year of life, paternal and maternal educational levels, household income, and paternal and maternal history of asthma, atopic eczema, and allergic rhinitis.
When children were stratified according to sex, an independent positive association was shown between PTB and wheeze only in male children: the adjusted ORs were 1.95 (95% CI: 1.37–2.75) and 0.90 (95% CI: 0.54–1.42) in male and female children, respectively; the multiplicative interaction between PTB and sex with regard to wheeze was significant (P for interaction=0.01). On the other hand, the adjusted ORs for asthma were 1.59 (95% CI: 0.97–2.50) and 1.42 (95% CI: 0.69–2.64) in male and female children, respectively (P for interaction=0.73).
DiscussionThe present study found that PTB was independently associated with a higher prevalence of wheeze in Japanese children aged three years, particularly in male children. Also, a significant positive relationship was observed between PTB and asthma, although sex did not modify the relationship. There were no evident associations between LBW, high birthweight, postterm birth, SGA, or LGA and wheeze or asthma.
The present results regarding birthweight are in partial agreement with those of a meta-analysis in 2015 showing a significant positive association between LBW and wheezing disorders and a null relationship between high birthweight and wheezing disorders4 but are at variance with a meta-analysis of European birth cohort studies which showed a significant positive association between LBW and wheezing disorders.5 With respect to recent studies that were not included in the abovementioned meta-analyses, a cohort study of 13,734 UK children found significant positive relationships between LBW and asthma diagnosis and wheezing symptoms between the ages of 0 and 7 years.11 In a cross-sectional study of 12,582 Brazilian infants aged between 12 and 15 months, LBW was significantly positively associated with the prevalence of wheezing once, recurrent wheezing, severe recurrent wheezing, hospitalization for wheezing, and diagnosis of asthma.12 A systematic review in 2016 suggested a positive association between LBW and childhood asthma or wheezing.13 The current results are inconsistent with these findings.
The current results regarding PTB are in agreement with those of two meta-analyses that reported a significant positive association between PTB and wheezing disorders.1,5 PTB was significantly associated with an increased risk of severe asthma in 1,760,821 children using data from Norwegian registries.14 The present results are in partial agreement with this finding. In a birth cohort study of 5915 US children, PTB was not significantly related to the risk of asthma during the first seven years of life.15 No associations were found between PTB and wheeze ever or asthma ever in a cross-sectional study of 6656 Iranian children ages 6–7 and 13–14 years.16 With respect to the interaction between PTB and sex, in a US prebirth cohort study, PTB was significantly positively associated with recurrent wheezing and doctor-diagnosed asthma during the first two years of life in girls, although no such positive associations were observed in boys.17 The current results are at variance with these findings.
A significant positive relationship was observed between SGA and childhood asthma in four studies conducted in Australia,3 Nordic countries,2 and Taiwan.6,7 The present results are inconsistent with these findings.
The observed positive associations between PTB and wheeze and asthma might be partly explained by lung function. In a meta-analysis using individual participant data of 24,938 children from 24 birth cohorts, compared with term-born children, those born preterm had lower FEV1, FEV1/FVC ratio, and FEF75.18 In this meta-analysis, PTB was significantly associated with an increased risk of childhood asthma as shown through mediation analysis: after additional adjustment for FEV1, FEV1/FVC ratio, or FEF75, the associations between PTB and asthma attenuated by −7% (95% CI: −19% to −1%), −14% (95% CI: −40% to −3%), and −39% (95% CI: −69% to −3%), respectively.18
The present investigation had methodological advantages in that study subjects were homogeneous in terms of age and in that reliable information on birthweight and gestational age at birth was drawn from data recorded by hospital or clinic staff in the Maternal and Child Health Handbook.
Important weaknesses of the present study should be considered. The participation rate was only 9.2%. The present study subjects were an unrepresentative sample of Japanese children in the general population, and the present findings may not be generalized. In fact, education levels were higher in our subjects’ parents than in the general population. According to the 2010 population census of Japan, the proportions of men 35–39 years of age in Fukuoka Prefecture with <13, 13–14, ≥15, and unknown years of education were 43.0%, 11.5%, 30.4%, and 15.1%, respectively.19 The corresponding figures among fathers in the present study were 30.7%, 14.6%, 54.8%, and 0.0%, respectively. The proportions of women 30–34 years of age in Fukuoka Prefecture with <13, 13–14, ≥15, and unknown years of education were 34.6%, 30.4%, 21.0%, and 14.0%, respectively.19 The corresponding figures among mothers in the present study were 23.6%, 33.6%, 42.7%, and 0.0%, respectively.
The definitions of wheeze and asthma were based on questions from the ISAAC although validation tests of these questions have not been performed for Japanese children aged three years. No attempt was made to ascertain outcome status through reviews of medical records. Data on IgE levels were not available in the present study. The consequence of inaccurate reporting of wheeze and asthma would have been an underestimation of values in our results because of non-differential outcome misclassification.
Although adjustment was made for several confounding factors, residual confounding effects could not be ruled out. Although a relatively large sample was used, the lack of significant positive associations between LBW and wheeze and asthma might be ascribed to insufficient statistical power.
To our knowledge, this is the first study in Japan to show that PTB was significantly positively associated with childhood wheeze and asthma. Findings from the present Japanese cross-sectional study suggest that LBW and SGA are not evidently related to childhood wheeze or asthma. Further studies are necessary to draw a firm conclusion on whether LBW, PTB, and SGA increases the risk of childhood wheeze and asthma, especially in Asian countries.
Conflict of interestThe authors have no conflict of interest to declare.
Ethical disclosureConfidentiality of dataThe authors declare that no subject's data appears in this article.
Right to privacy and informed consentThe authors have obtained the informed consent of the parents or guardians.
Protection of human subjects and animals in researchThe authors declare that no experiments were performed on humans or animals for this investigation.
The authors thank the municipal governments that supported the KOCHS and all study participants.
This study was supported by JSPS KAKENHI (Grant number JP24390158). This organization did not have any influence on the study design; the collection, analysis, or interpretation of data; the writing of the report; or the decision to submit the article for publication.