Polymorphonuclear neutrophils (PMNs) were originally described as short lived and terminally differentiated phagocytes that contribute only to the innate immune response. Some studies of PMNs cytokine production and expression of numerous cell surface proteins has suggested that PMNs are likely to influence adaptive responses and may satisfy the criteria of antigen presenting cells.
Aim of the studyThis work aimed to study the effect of IL-4 in the function of PMNs as antigen presenting cells.
MethodsFlow cytometry was used in the present study for the detection of cell surface human leukocyte antigen (HLA) class II, CD80 and CD86 required for antigen presentation and subsequent T-cell activation in the presence of Staphylococcus aureus enterotoxin (A). Human peripheral blood neutrophils were used for this purpose.
ResultsThis study has shown that IL-4 stimulated PMNs for 24h expressed HLA class II, CD80 and CD86 that involved in antigen presentation. It also indicated that co-cultivation of IL-4 stimulated PMNs with autologous T-cells and in the presence of S. aureus enterotoxin (A) induced T-cell proliferation.
ConclusionsIn vitro stimulation of PMNs with IL-4 showed expression of surface molecules involved in antigen presentation. In addition, the co-culture of T-Cells and stimulated PMNs showed high T-Cells proliferation in the presence of superantigens.
Polymorphonuclear neutrophils (PMNs) possess a very short half-life in the circulation because they constitutively undergo apoptosis.1,2 Under certain conditions PMNs play an important role in the effectors arm of host immune defence through the clearance of immune complexes, the phagocytosis of opsonised particles, and the release of inflammatory mediators.3–5 During the last years the image of PMNs has changed considerably. Traditionally considered to be the first line of defense against bacterial infection, it became increasingly clear that PMNs also participate in chronic inflammation disease and regulation of the immune response when appropriately activated.6 For surface molecules, there are several reports that PMNs from a variety of species can express human leukocyte antigen (HLA) class II and costimulatory molecules (CD80 and CD86).7–10 Under certain stimulation murine neutrophils present HLA class II restricted antigen.11
PMNs function and recruitment to the site of inflammation have been shown to be upregulated by various cytokines, including interleukin (IL)-1, IL-8, tumour necrosis factor (TNF), interferon-γ (IFN-γ), and granulocyte macrophage-colony stimulating factor (GM-CSF).5,12 In addition, IL-15 stimulated PMNs acquire HLA-DR.13
IL-4 production has been found to occur in thymocytes, mature T-cells, certain malignant T-cells, mast-cells and basophiles and occasionally, in transformed B-cells.14 It has an effect on B-cells, T-cells, monocytes, mast-cells, endothelial cells, and fibroblasts.15 Directly and/or indirectly, IL-2 has a prominent role in the regulation of IL-4 producing cells.14 IL-4 binds to a high-affinity cell-surface receptor (IL-4R) to exert its effects.16 It promotes the growth and differentiation of activated human B-lymphocytes and shares many biological functions with IL-13.17
Following this approach, the present study was aimed to estimate the expression of the antigen presenting molecule HLA class II and the co-stimulatory molecules CD80 and CD86 on PMNs stimulated with IL-4. The expression of these surface molecules prompted us to test the T-cell proliferation by their co-culture with IL-4 stimulated PMNs in the presence Staphylococcus aureus enterotoxin (A) as a superantigen.
Materials and methodsBlood was taken from 10 healthy Egyptian people by venous puncture using 7.5ml heparin-coated tubes (Sarstedt, Nümbrecht, Germany) and was analysed within 2h. Recombinant human (rh) IL-4 was purchased from Sigma (St Louis, MO, USA). For fluorescence-activated cell sorter (FACS) analysis of whole blood, erythrocyte FACS lysing solution was obtained from Becton Dichinson (Heidelberg, Germany) and diluted 1:10 in bidistilled water. For cytofluorometry fluorescein isothiocyanate (FITC) and phycoerythrin (PE)-labelled murine MoAbs were used. Mouse IgG1 FITC, IgG2a PE, CD66b-FITC, HLA-DP+DQ+DR:PE, CD80:PE and CD86:PE were obtained from Coulter Immunotech (Marseille, France).
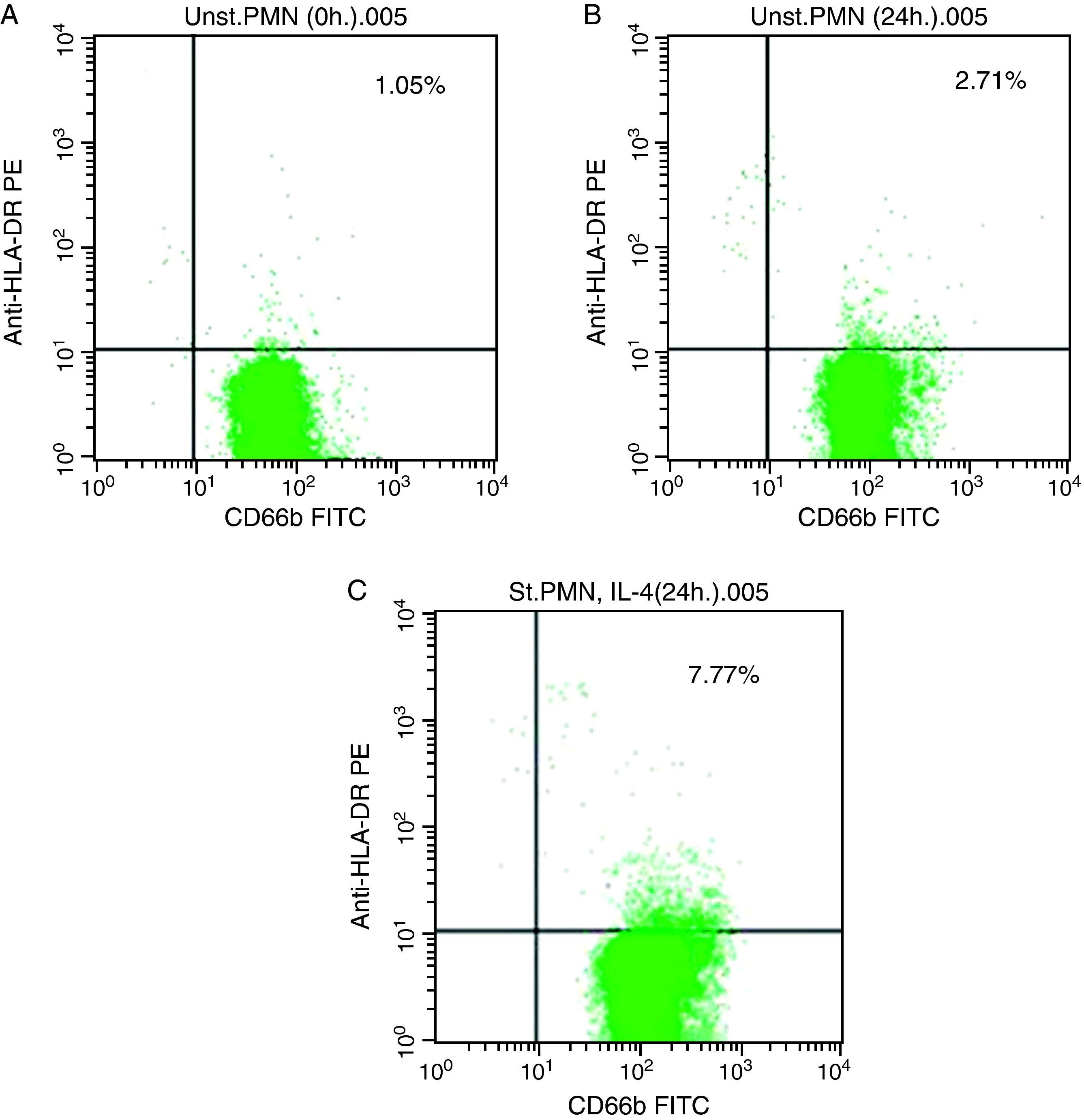
For double labelling, anti-CD66b-FITC as a PMNs marker and the respective PE-labelled antibody were used in equal protein concentrations. Cells in whole blood were analysed by FACSCalibur and CellQuest software (Becton Dickinson, SanDiego, CA, USA). Results were expressed as the percentage of positive cells in the respective gate or quadrant. In FACS plots, there are different populations. So, gates were made around population with high CD66b-FITC.
In all FACS experiments, PMNs in heparinised blood were cultured in 24-well plate, 2ml/well and incubated in the presence or absence of IL-4 (6ng/ml) for about 24h at 37°C with 5% CO2.
For the co-culture of T-cells and PMNs, cells were isolated by Polymorph Prep® (Nycomed; Oslo, Norway). PMNs and T-cells fraction were further purified by adsorption to CD15 and CD3 beads (Miltenyi Biotec; Bergisch Gladbach, Germany), respectively, by magnetic cell separation using the devices supplied by Milteny Biotech (Bergisch-Gladbach, Germany).
Highly purified PMNs (1×106/ml) were cultivated in AIM V (Gibco BRL; Paisley, Scotland)) with 2.5% autologous normal human serum, NHS (inactivated at 56°C for 30min.). T-cells were cultured in RPMI 1640 (Gibco BRL; Paisley, Scotland) supplemented with 10% fetal calf serum (FCS) (PAN Biotech GmbH; Aidenbach, Germany), 100U/ml penicillin/streptomycin (Gibco BRL; Paisley, Scotland), 2mM l-glutamine (Gibco BRL; Paisley, Scotland)), and 10mM HEPES (Gibco BRL; Paisley, Scotland). All cells were incubated at 37°C and 5% CO2 for the times indicated.
Unstimulated and stimulated PMNs (1×103) in 100μl were added per well of a 96-well concave-bottom plate (Greiner; Nuertingen, Germany). Then, 1×104 T-cells (100μl) were added to each well together with 25ng S. aureus enterotoxins A (Sigma; Munich, Germany). After coincubation for four days at 37°C with 5% CO2, proliferation was tested by adding 1mCi of 3H-thymidine (Amer-sham Life Science; Braunschweig, Germany) for 6–8h [3H] TdR incorporation into DNA was measured and expressed as counts per minute (cpm). The values represent the mean±SE of 6–12 parallel wells.
Statistical analysis of the obtained data was performed using one way analysis of variance (ANOVA) test followed by least square differences (LSD) analysis for comparison between means. Results were expressed as mean±standard error (SE), and differences were considered to be significant at P<0.05.
ResultsResults were expressed as percentage of positive cells in the respective gate or quadrant. Each set of experiments was repeated in vitro ten times.
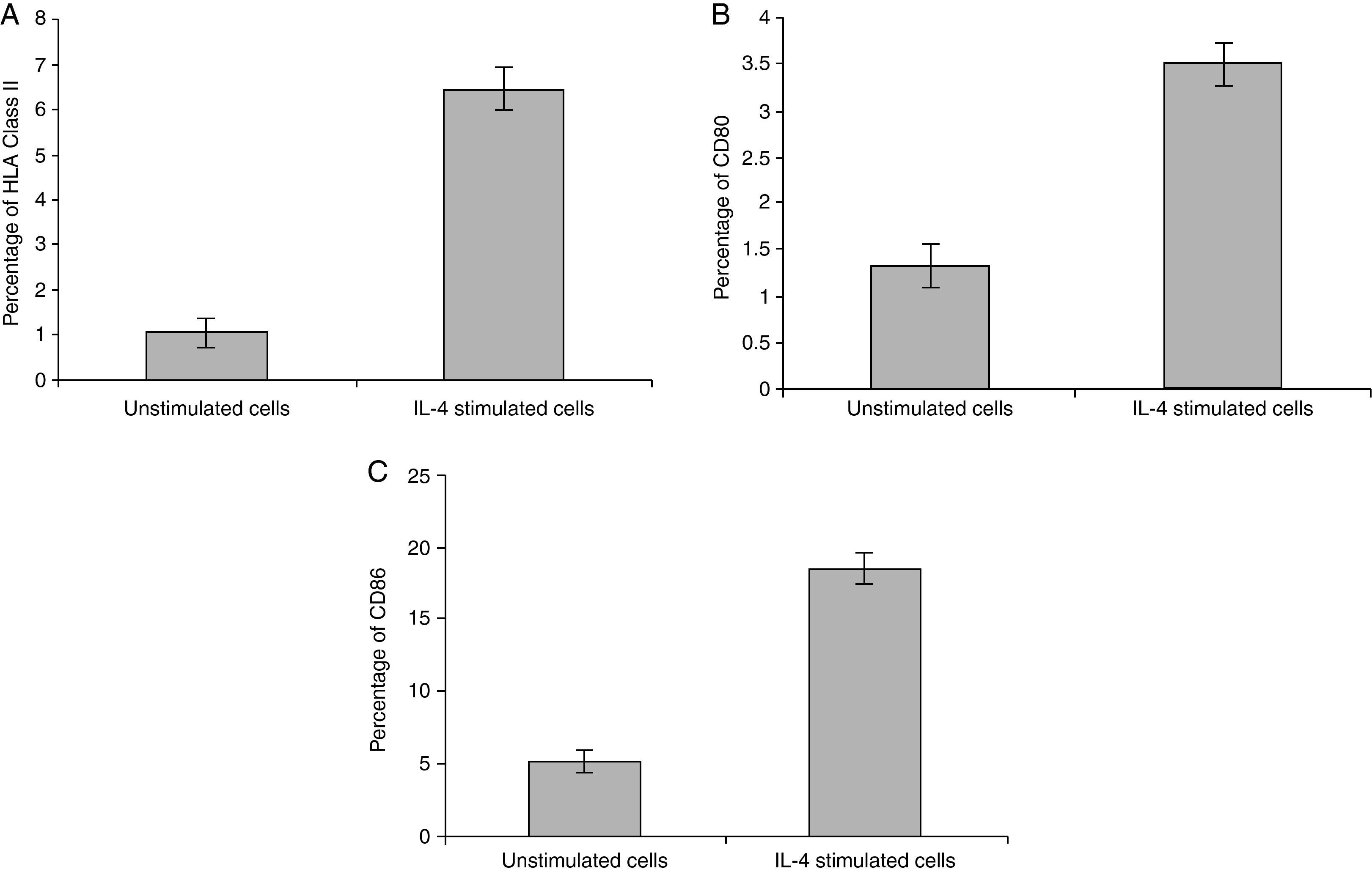
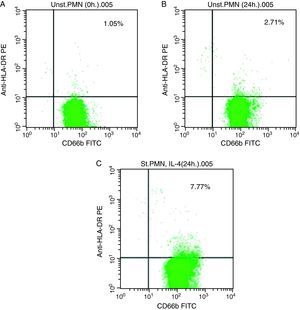
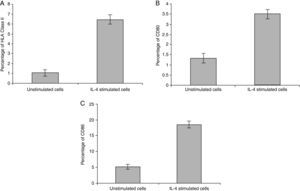
In vitro expression of HLA class II on PMNsThe majority of healthy donors PMNs expressed CD66b (Fig. 1a–c). In the first set of experiments the effect of IL-4 on the expression of HLA class II was tested, where we found that PMNs on whole blood showed expression of HLA class II recording 7.77% (Fig. 1c) by using IL-4, as stimulators. In contrast, fresh and unstimulated cells cultured with medium only counted 1.05% (Fig. 1a) and 2.71% (Fig. 1b), respectively.
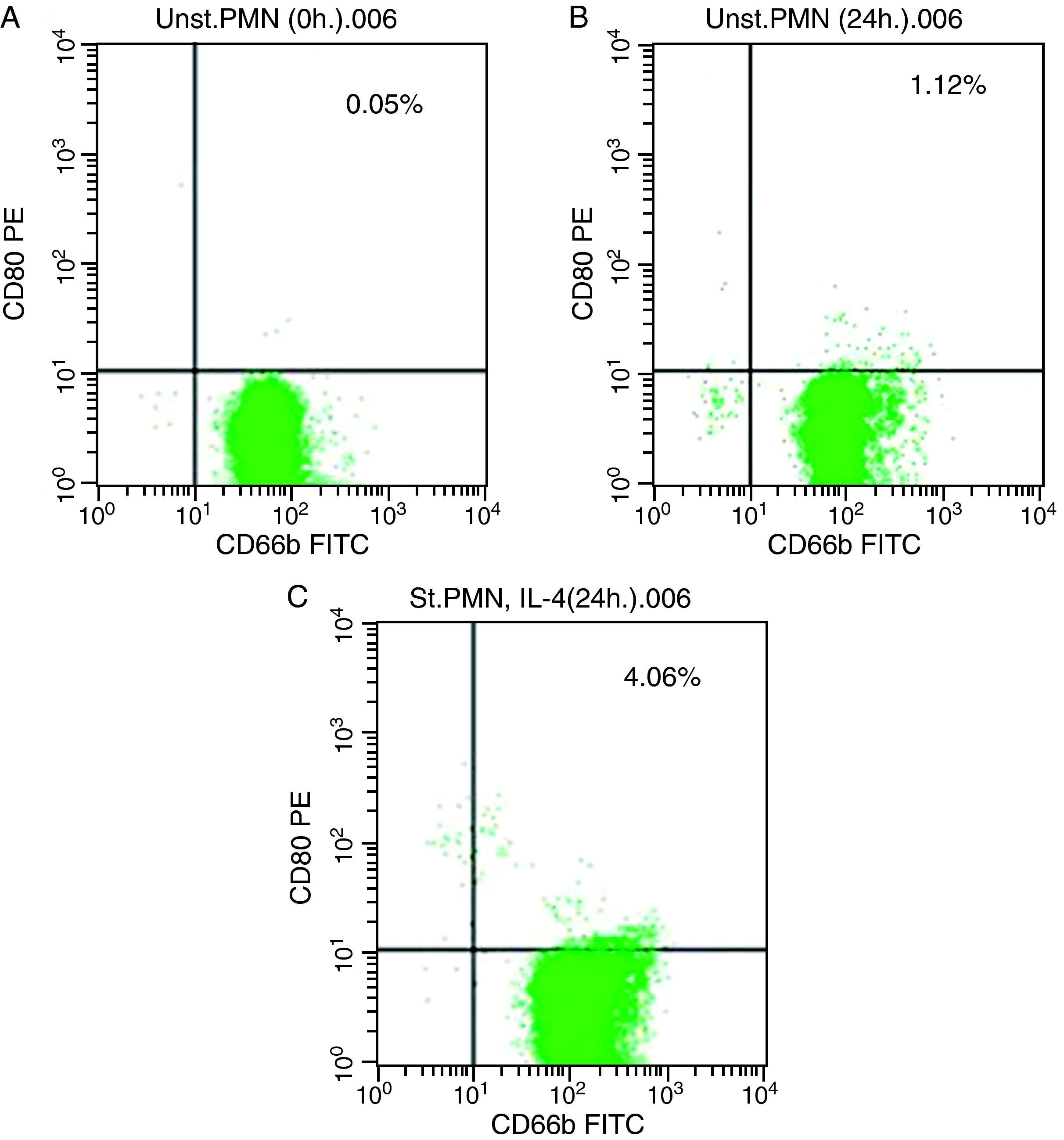
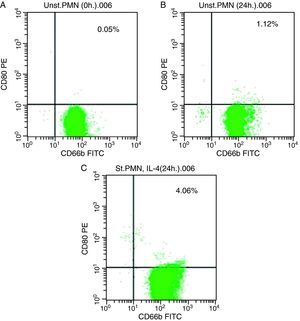
In vitro induction of CD80 on PMNsAs shown for HLA class II, surface expression of CD80 was most impressive following cultivation of whole blood with the stimuli for 24h. The proportion of double-positive cells (right upper quadrant) was estimated, where CD80 positive cells was slightly higher in stimulated cells recording 4.06% by using IL-4 (Fig. 2c) than unstimulated PMNs cultured with medium for 24h (1.12%) and fresh cells, 0.05% (Fig. 2a and b, respectively).
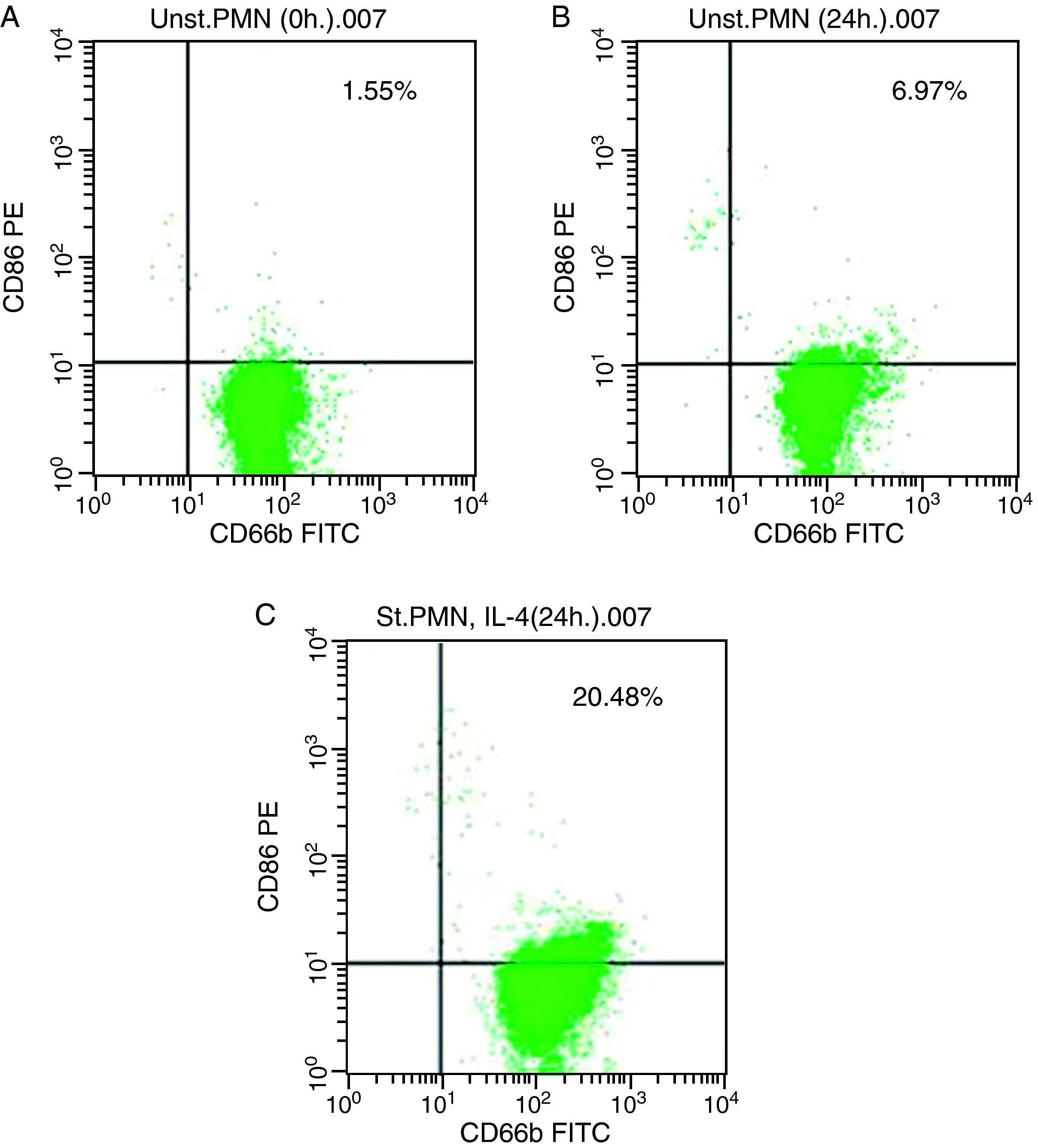
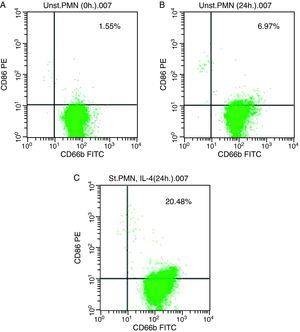
In vitro induction of CD86 on PMNsFor CD86, high expression was recorded on the surface of IL-4 stimulated PMNs in whole blood recorded 20.48% (Fig. 3c), while percentage of CD86 molecules on the surface of unstimulated PMNs measured 6.97 (Fig. 3b). Fresh PMNs in while blood had 1.55% (Fig. 3a).
Statistical analysis of HLA class II, CD80 and CD86 experimental sets showed that there is a significant difference between IL-4 stimulated cells and unstimulated cells, P<0.05 (Fig. 4).
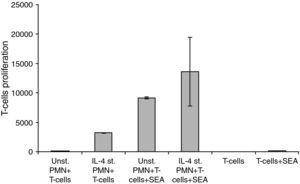
Interaction of IL-4 stimulated PMNs with peripheral T-cellsFor these experiments highly purified PMNs were cultivated with IL-4 for 24h and then co-cultivated with highly purified isolated T-cells in the absence or presence of SEA. The differences between the groups in the sets of experiments related to the interaction of HLA class II positive PMNs with peripheral T-cells showed a significant difference, where P<0.05 (Fig. 5).
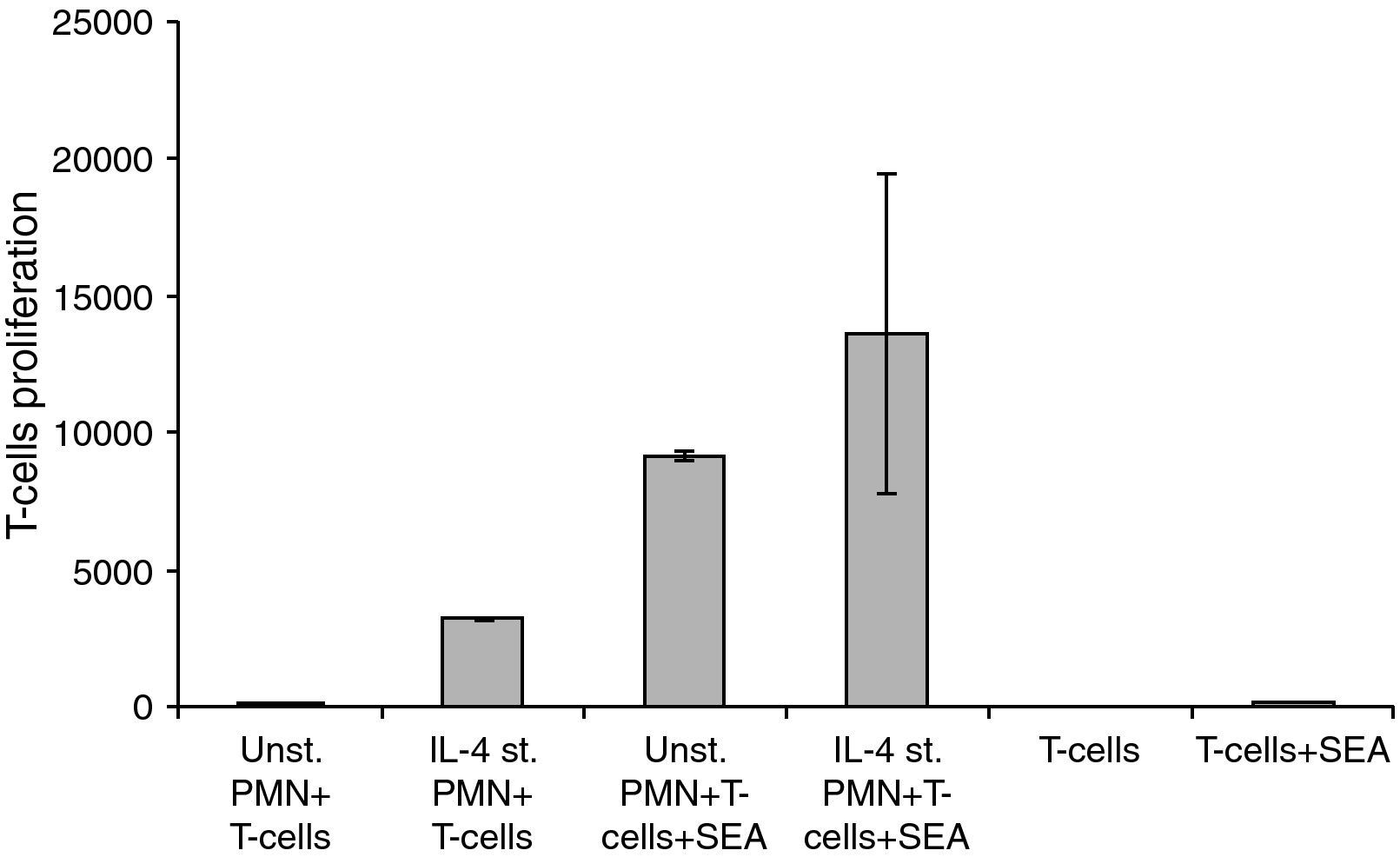
Changes in the proliferation of T-Cells co-cultured with either unstimulated or IL-4 stimulated PMN in the presence and absence of superantigen. Statistical analysis showed that, there is a significant difference in T-Cells proliferation between T-Cells co-cultured with IL-4 stimulated and unstimulated PMN in superantigen presence, where P<0.05.
PMNs are considered short-lived cells undergoing spontaneous apoptosis in vivo as well as in culture.18 Previous studies have demonstrated that PMNs can be induced in vitro to synthesize and release various cytokines, suggesting that these cells can contribute significantly to the initiation and amplification of cellular and humoral immune responses.5 The detection of these molecules, therefore, provides strong support for the hypothesis that human PMNs can actively synthesize immunoregulatory molecules19 and have the potential to act as antigen presenting cells.8
Recently, it has become increasingly evident that culturing PMNs in the presence of cytokines extends their life span.20–23 Cultured PMNs synthesize and release immunomodulatory cytokines by which they may participate in the afferent limb of the immune response.5
This study has clearly shown that PMNs could be induced to express HLA class II, CD80 and CD86 after activation with IL-4. These data are in accordance with other results where HLA class II, CD80 and CD86 were expressed on the PMNs surface after exposure to GM-CSF and/or INF-γ.6
The antigen presenting molecule HLA class II and the co-stimulatory molecules CD80 and CD86 play an important role in T-cell proliferation, where HLA class II presents the engulfed antigen to T-cells.6 CD80 and CD86 act as second signal molecules involving in the stimulation of T-cells to produce the autocrine growth factor IL-2 without which T-cells are thus unable to proliferate.24
The activation and recruitment of PMNs were also regulated by IL-15,13 IFN-γ,25 CSF-CSF26–28 and IL-8.29 In addition, PMNs posses IL-2Rβ chain30,31 and IL-2Rγ chain32 that have the ability to bind with IL-4.33 These published data prompted us to study the effect of IL-4 on PMNs functions.
When comparing the number of monocytes and PMNs required to induce T-cell proliferation, it was observed that 10 times more PMNs than monocytes were necessary to yield the same extent of T-cell proliferation. However, the cell preparation used in this work never contained more than 1% of contaminating cells and certainly not the 10% of monocytes that would be required to affect the results.30 The observation that ten times more PMNs than monocytes were required to induce a similar extent of T-cell proliferation has to be considered together with the observation that only a proportion of PMNs acquired HLA class II. Thus, when only fully equipped PMNs are considered, the ability to process and to present antigen is similar to that of monocytes. Whether HLA class II-positive PMNs participate in the immune defence or play a role in pathophysiological events, is a matter of speculation. The fact that only a minor proportion of PMNs acquire HLA class II might lead to the conclusion that a possible accessory function of PMNs would be rather weak. One has; however, to bear in mind that PMNs are numerous in the peripheral blood, and that even a low percentage of PMNs expressing HLA class II would exceed both circulating monocytes and dendritic cells in number.
Due to the notion that the presence of Staphylococcus entrotoxin coincides with relapses of Wegener's granulomatosis,34 we tested whether PMNs was able to also present Staphylococcus enterotoxin A as a superantigen, so-called because it binds outside of the peptide-binding groove of the HLA class II and the antigen-specific domain of the T-cell receptor, and consequently activates a large portion of T-cells, preferentially those with a V beta 2 domain.35 In accordance with previous studies,8,36 co-culture of PMNs with T-cells and SEA resulted in T-cell proliferation. Taken together, our data demonstrate that by synthesizing and expressing of HLA class II antigens, CD80 and CD86; PMNs acquire the capacity to present superantigens to T-cells.
Data showed that there is a clear increase in T-lymphocyte proliferation in the co-culture of stimulated PMNs+T-cells. Because there is no antigen present, this means that it is not caused by the co-stimulatory molecules on the surface of the PMNs but probably due to mediators secreted by the PMNs. Furthermore, unstimulated PMNs+T-cells+SEA give almost a 1000-fold increase of T-cell proliferation while only a few percent of PMNs express co-stimulatory molecules in a naive state. Probably the results obtained are both from released mediators and the co-stimulatory molecules. Mediators secreted by IL-4 stimulated PMNs will be investigated in our future research.
In conclusion, stimulated PMNs with IL-4 lead to expression of the antigen presenting molecules (HLA class II) and the co-stimulatory molecules (CD80 and CD86). These molecules play an important role in antigen presentation and consequently T-cell proliferation in the presence of SEA. This means that IL-4 stimulated PMNs might be involved indirectly in acquired immune response in addition to their role in innate immunity.
Conflict of interestThe authors have no conflict of interest to declare.
I thank Prof. Dr. El-Feki M.A. (Zoology Department, Faculty of Science, El-Minia University, El-Minia, Egypt) for revising the manuscript. I also thank Dr. Shaban H.A. (Zoology Department, Faculty of Science, El-Minia University, El-Minia, Egypt) for his efforts in data analysis.