Erythropoietin (EPO) is originally defined as a haematopoietic growth factor, but also has anti-inflammatory effects through cytokine modulation. This anti-inflammatory and cytokine modulating effect has not been investigated for the treatment of asthma. We aimed to determine the beneficial effects of erythropoietin on lung histology of murine model of chronic asthma.
MethodsThirty-five BALB/c mice were divided into five groups: I; II; III; IV; and control group. All groups except control group were sensitised and challenged with ovalbumin. Mice with experimentally induced asthma in Group I received saline; Group II EPO 500IU/kg; Group III EPO 1000IU/kg; and Group IV dexamethasone 1mg/kg intraperitoneally once a day in the last five days of the challenge period. Animals were sacrificed 24h after the last administration of study drugs. Histological findings of airways were evaluated by light and electron microscopic examination.
ResultsAll histological parameters of asthma in the group treated with a high dose of EPO (Group III) were significantly ameliorated when compared with the group treated with saline (Group I). In comparison to the group treated with low dose of EPO (Group II) and the group treated with saline (Group I), basement membrane thicknesses and number of mast cells were significantly lower in the group treated with low dose of EPO (Group II). All histological parameters were similar between the group treated with high dose of EPO (Group III) and the group treated with dexamethasone (Group IV) except higher number of mast cells in the group treated with high dose of EPO (Group III). Additionally, the results of all histological parameters in the group treated with high dose of EPO (Group III) were significantly better when compared with the group treated with low dose of EPO (Group II).
ConclusionsWe found that EPO ameliorated histological changes of chronic murine model of asthma. Further studies are needed to evaluate the efficacy of EPO in the treatment of asthma.
Asthma is a chronic disease characterised by reversible airway obstruction, airway inflammation, and remodelling.1 Current strategies for the management of asthma focus on suppressing airway inflammation, the key factor in the pathogenesis of asthma.2 Airway remodelling, a potentially important consequence of asthma, comprises progressive structural changes in the composition, content, and organisation of the cellular and molecular constituents of the airway wall, as well as enhanced turnover of extracellular matrix components.3 It includes goblet cell hyperplasia, basement membrane thickening, subepithelial fibrosis, airway smooth muscle hypertrophy/hyperplasia, and angiogenesis.4 Airway remodelling is poorly responsive to current anti-inflammatory asthma therapies, including inhaled corticosteroids and antileukotrienes.5,6 Thus, new therapeutic options are required.
Erythropoietin (EPO), a 30.4-kD glycoprotein that regulates the rate of red blood cell production through binding to its specific cell surface receptors, has been used for many years for the treatment of anaemia.7 EPO has pleiotropic actions including antioxidant, anti-apoptotic, anti-inflammatory and angiogenic effects. Antioxidant effect of EPO is via decreasing plasma iron concentration and increasing the capability of plasma in inhibition of lipid peroxidation.7–10 EPO also inhibits the synthesis of IL-4, IL-5.11 Thus, given the abovementioned effects, EPO may have a role in the treatment of asthma. However, the accurate benefits of EPO in asthma treatment have not been investigated yet. Therefore, in this study our aim is to investigate the efficacy of EPO on lung histopathology in a murine model of chronic asthma.
Materials and methodsExperimental animalsSpecific pathogen-free, 6- to 8-week-old, female BALB/c mice, weighting 18–20g, were maintained in a pathogen-free laboratory of Dokuz Eylul University. They were kept in hygienic macrolene cages in air-conditioned rooms and allowed ad libitum with food and water on a 12-h light/12-h dark cycle. All experimental procedures complied with the requirements of the Animal Care and Ethics Committee of the Dokuz Eylul University. Thirty-five BALB/c mice were divided into five groups: I, II, III, IV, and control group, each group included seven mice.
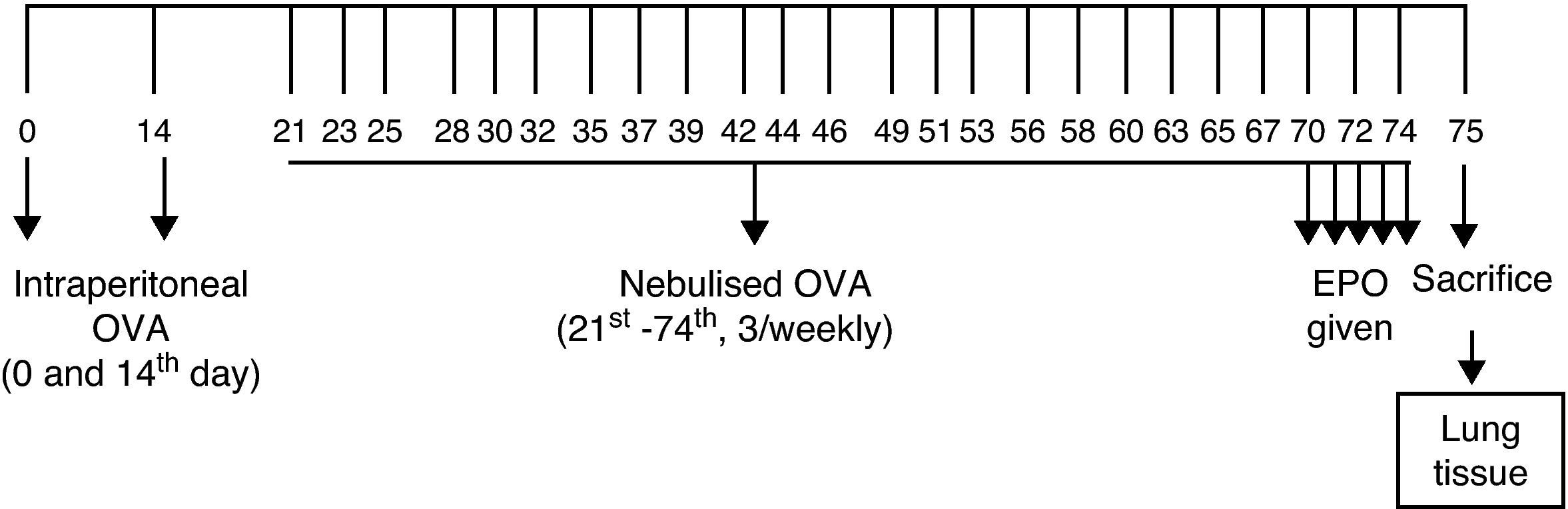
Sensitisation and inhalational exposureBALB/c mice are high responders to ovalbumin.12 The mice in study groups I, II, III, and IV were sensitised via two intraperitoneal injections, on days 0 and 14 of the experiment, of 10μg/0.1mL chicken egg albumin (ovalbumin, grade V, ≥98% pure; Sigma, St. Louis, MO, USA) with alum as an adjuvant. The mice in study groups I, II, III, and IV were then exposed to aerosolised ovalbumin for 30min per day on three days of a week for eight weeks, beginning from the 21st day of the study (Fig. 1). Exposures were carried out in a whole body inhalation exposure system. Temperature and relative humidity were maintained between 20–25°C and 40–60%, respectively. A solution of 2.5% ovalbumin in normal saline was delivered by aerosolisation via compressed air to a sidestream jet nebuliser injected into a chamber. The aerosol generated by this nebuliser comprised >80% particles with a diameter of <4μm. Particle concentration was maintained in the range of 10–20mg/mm3.13
The mice in the control group were administered normal saline with alum via intraperitoneally on the 0 and 14th days of the experiment and exposed aerosolised saline for 30min per day on three days of the week for eight weeks, beginning from the 21st day of the study.12,13
Study drugsMice in Group I received saline; Group II received EPO at dose of 500IU/kg; Group III received EPO at dose of 1000IU/kg; and Group IV dexamethasone at dose of 1mg/kg intraperitoneally once a day in the last five days of the challenge period. Intraperitoneal doses of human recombinant EPO (Epoetin alpha, Eprex, Roche) and dexamethasone 1mg/kg were chosen from other studies also conducted with BALB/c mice.13–15
Histopathological analysisAnimals were sacrificed by an overdose of ketamine 24h after the last drug administration. Two investigators who were blinded to the treatment groups interpreted the histopathology. Tissue specimens were obtained from the mid zone of the left lung of mice. Samples were fixed in 10% formalin for light microscopic evaluation. Some tissue samples of 1–2mm3 obtained from adjacent regions were stocked in 2.5% gluteraldehyde for electron microscopic evaluation. After fixation, samples were embedded in paraffin for light microscopic evaluation and serial sections of 5-μm thickness were prepared. After choosing the first section randomly, 10 sections in each mouse were selected by skipping over 10 sections and proceeded to staining process. For light microscopic evaluation, three different staining processes were used. The first 10 samples were stained with haematoxylin and eosin (H&E). In these samples, general tissue features were examined and thicknesses of epithelium and subepithelial smooth muscle layers of the medium and small airways were measured. In order to evaluate the thicknesses of epithelium and subepithelial smooth muscle layers, measurements were performed from four points of each airway at levels of 3, 6, 9, and 12o’clock. Considering that each section contained approximately two to three airways, around 20 or more airways were evaluated for each mouse.
Photomicrographs were taken by JVC TK-890-E camera (Japan), which was adapted on Olympus BH-2 RFCA model microscope (Olympus Optical, Tokyo, Japan). The histological analysis was carried out with UTHSCSA Image Tool for Windows Version 3.00 software.
The consecutive 10 sections were stained with toluidine blue and the other 10 sections with periodic acid-Schiff (PAS). Photomicrographs were taken randomly from five fields of each section which were stained with toluidine blue. For mast cell enumeration, a standard transparent counting frame representing an area of 20,000μm2 was used manually and eight fields in each photograph were examined for each mouse. Goblet cells stained with PAS were enumerated in 10 sections of each mouse. In each section, three to five randomly selected airways were photographed. Circumferences of all airways were measured and goblet cell numbers in these areas were recorded. For standardisation, goblet cell numbers in 100μm were analysed by division of total goblet cell number to the total length of airway circumferences and multiplying the result by one hundred.
Tissues were embedded in EPON after follow-up process of electron microscopic evaluation. Airways were marked from the semithin sections by light microscope. Ultrathin sections were obtained and stained with uraniyl acetate and lead citrate. Libra 120 Carl Zeiss EFTEM electron microscope (Oberkochen, Germany) was used for this evaluation. For each mouse, five to seven ultrathin sections were achieved from each two blocks for evaluation of epithelium of the airway, the surrounding structures, and the intercellular connections.
For each mouse, eight to ten areas were photographed by Trondle (2048×2048 pixel) digital camera, attached to the electron microscope. Thicknesses of the basement membrane of the respiratory epithelium were measured from 20 points of preparations at equal distances to each other and the data were recorded in sequence.
Statistical analysisSPSS 11 package program was used for the statistical analysis. Data were presented as mean±standard deviation (SD) (minimum–maximum) of seven animals in each group. The comparisons between all groups were conducted by using Kruskal–Wallis method. When differences were statistically significant, Mann–Whitney U test was used for group comparisons. p<0.05 was considered statistically significant.
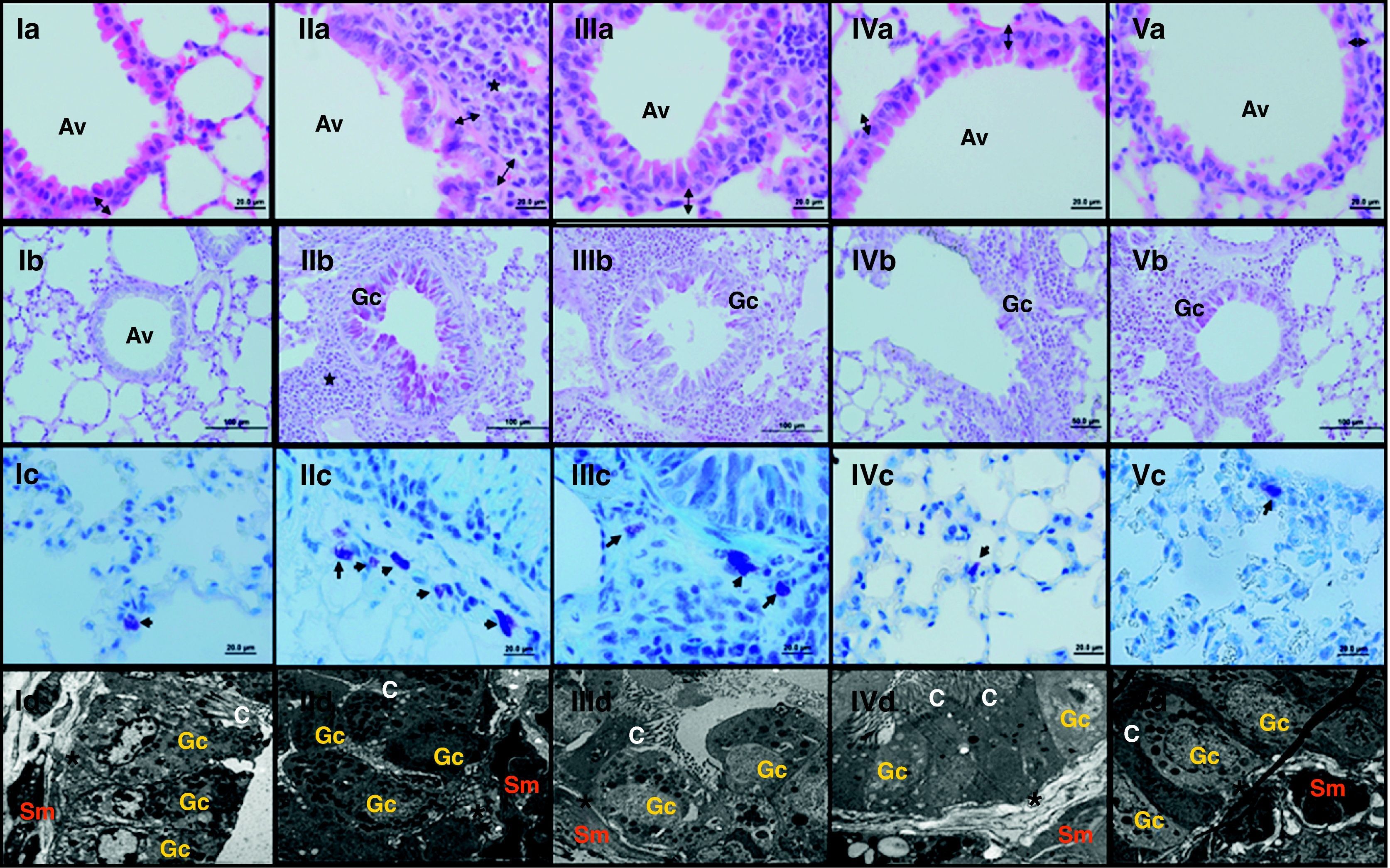
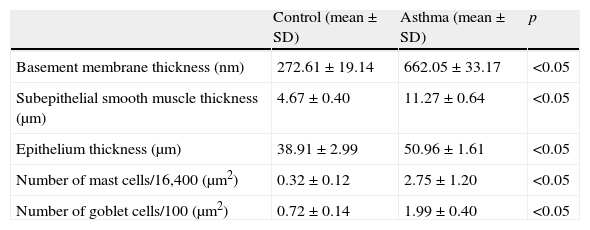
ResultsThe light and electron microscopic examinations revealed normal findings in the control group (Fig. 2-I). In the chronic asthma group (placebo), the numbers of mast cells and goblet cells as well as the thicknesses of basement membrane, epithelium, and subepithelial smooth muscle layer were significantly higher when compared to the control group (Table 1). Additionally, Fig. 2-II demonstrates that characteristic asthmatic changes were successfully established in the group treated with saline (Group I).
Light and electron microscopic findings of Groups. I; control, II; asthma, III; EPO (500IU/kg), IV; EPO (1000IU/kg), V; Dexa group. In representative histological images, lung tissues were stained with H&E (a; 1st row), PAS (b; 2nd row) and toluidine blue (c; 3rd row). In control group; light microscopic findings (Ia) revealed a regular respiratory epithelium and airways (Av), a thin, regular subepithelial smooth muscle layer (arrow with two heads), and normal PAS-stained parenchymal structures (Ib). Parenchymal structures with toluidine blue staining were regular (Ic). Electron microscopy (Id) revealed goblet cells (Gc), and healthy epithelial cells with cilia (C). The basement membrane was thin and regular (*), and there were two to three layers of subepithelial smooth muscle cells (Sm). In asthma group; H&E staining (IIa) revealed thickened epithelium, thickened subepithelial smooth muscle (arrow with two heads), and peribronchial mononuclear infiltration (*). High numbers of goblet cells (Gc) were seen with PAS-staining (IIb), and mast cells with toluidine blue staining (IIc). In dexa group; no difference in light microscopic findings was found compared with control rats (Va, Vb, Vc and Vd), In EPO (500IU/kg) and EPO (1000IU/kg) groups; the pathological changes, cellular infiltration around airways, goblet cell hyperplasia and number of mast cells, were significantly less than in asthma group. Electron microscopic findings revealed a thickened basement membrane (*) and smooth muscle cells (Sm) in asthma group (IId). Healthy respiratory epithelium with cilia (C) and goblet cells (Gc) in EPO (500IU/kg) (IIId), EPO (1000IU/kg) (IVd) and dexa (Vd) groups.
Comparison between asthma (Group I) and control groups.
| Control (mean±SD) | Asthma (mean±SD) | p | |
| Basement membrane thickness (nm) | 272.61±19.14 | 662.05±33.17 | <0.05 |
| Subepithelial smooth muscle thickness (μm) | 4.67±0.40 | 11.27±0.64 | <0.05 |
| Epithelium thickness (μm) | 38.91±2.99 | 50.96±1.61 | <0.05 |
| Number of mast cells/16,400 (μm2) | 0.32±0.12 | 2.75±1.20 | <0.05 |
| Number of goblet cells/100 (μm2) | 0.72±0.14 | 1.99±0.40 | <0.05 |
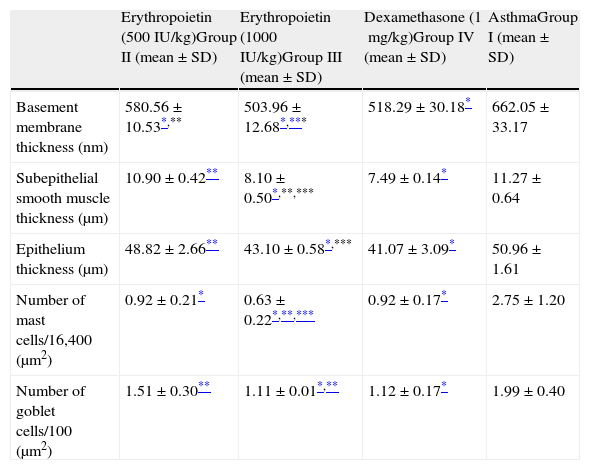
In Group II (EPO 500IU/kg), basement membrane thickness and number of mast cells were significantly lower compared with regard to the group treated with saline (Group I). Both the thicknesses of epithelium and subepithelial muscle layer and the number of goblet cells were similar to that of the group treated with saline (Group I). Additionally, respiratory tract epithelium and ciliary structures were similar to normal airway findings (Table 2 and Fig. 2-III).
Comparison between study groups.
| Erythropoietin (500IU/kg)Group II (mean±SD) | Erythropoietin (1000IU/kg)Group III (mean±SD) | Dexamethasone (1mg/kg)Group IV (mean±SD) | AsthmaGroup I (mean±SD) | |
| Basement membrane thickness (nm) | 580.56±10.53*,** | 503.96±12.68*,*** | 518.29±30.18* | 662.05±33.17 |
| Subepithelial smooth muscle thickness (μm) | 10.90±0.42** | 8.10±0.50*,**,*** | 7.49±0.14* | 11.27±0.64 |
| Epithelium thickness (μm) | 48.82±2.66** | 43.10±0.58*,*** | 41.07±3.09* | 50.96±1.61 |
| Number of mast cells/16,400 (μm2) | 0.92±0.21* | 0.63±0.22*,**,*** | 0.92±0.17* | 2.75±1.20 |
| Number of goblet cells/100 (μm2) | 1.51±0.30** | 1.11±0.01*,** | 1.12±0.17* | 1.99±0.40 |
When EPO was administered at a dose of 1000IU/kg (Group III), significant improvement occurred in all histological parameters compared with the group treated with saline (Group I). This improvement was similar to dexamethasone, the gold standard treatment of asthma (Table 2). Parenchymal structures and respiratory epithelium were normal (Fig. 2-IV).
In Group IV (dexamethasone 1mg/kg), all histological parameters were significantly better compared to the group treated with saline (Group I). Electron microscopic findings demonstrated regular basement membrane and normal ciliary epithelium (Table 2 and Fig. 2-V).
When low and high dosages of EPO were compared to each other; all histological parameters were found to be significantly better in the group treated with high dose of EPO (Group III) (p<0.05) (Table 2). When EPO groups were compared with dexamethasone group, among all the histological variables, the only significant difference was lower mast cell counts in the group treated with high dose of EPO (Group III) (p<0.05) (Table 2).
DiscussionAirway inflammation in asthma causes subsequent structural changes called as remodelling.3 Structural changes in asthmatic airways occur as a result of an injury/repair process. Currently, drugs used for the treatment of asthma have little effect on airway remodelling.16 EPO has uncertain value in the treatment of asthma. Although the anti-inflammatory properties of EPO have been reported many times, their effects on remodelling have rarely been evaluated. Thus, we aimed to investigate the efficacy of EPO on lung histological changes and especially on remodelling in a murine model of chronic asthma.
Chronic inflammation in asthma is thought to initiate and perpetuate tissue injury and repair.17 In our study, the structural changes observed in the asthmatic group revealed that the model was well established. It seems very important to prevent airway remodelling in the chronic management of asthma,3 because once formed, remodelling is resistant to asthma therapy.18 Inhalation of corticosteroids and administration of β2 agonists, antileukotrienes, and theophylline are poorly responsive to these structural changes. Inhaled corticosteroids may be effective in reducing reticular basement membrane thickness when used for a long period of time and at high doses.5 In our study, dexamethasone and high dose of EPO significantly ameliorated all the structural changes of chronic asthma. This finding is very important because reticular basement membrane thickness is considered a hallmark for airway remodelling in asthma.19
EPO is a hormone which maintains erythrocyte mass. The major mechanism for increasing in erythrocyte mass is the prevention of apoptosis of late erythroid progenitor cells.20 In the haematopoietic system, the principal function of EPO is the regulation of red blood cell production, mediated by its specific cell surface receptor. Recombinant EPO forms EPO alfa, EPO beta, and long-acting analogue darbepoetin alfa have been widely used for treatment of anaemia in chronic kidney disease and chemotherapy-induced anaemia in cancer patients.21
Recent studies showed that EPO protects various tissues through antiapoptotic effects. EPO protects the developing brain against N-methylaspartate receptor antagonist induced neuronal cell apoptosis,22 and inhibits spinal neuronal apoptosis and pain following nerve root crush.23 EPO also markedly reduced apoptosis of adult rat cardiomyocytes subjected to hypoxia in vitro, and reduced cardiomyocyte loss by 50%.24 In addition, EPO protected tubular epithelial cells against apoptosis induced by ischemic acute renal injury.25
EPO is a complex anti-inflammatory agent which exerts a wide variety of immunosuppressive effects targeting epithelial cells, neutrophils, T and B lymphocytes, mast cells, natural killer cells, and endothelial cells. Additionally, EPO has an inhibiting effect on the synthesis of some inflammatory cytokines such as IL-4, IL-5.11 IL-4, IL-5 are important mediators in pathogenesis of asthma. We considered that EPO, especially at a higher dose, showed beneficial results owing to its anti-inflammatory and anti-reactive oxygen species effects. Due to the delayed expression of EPO gene after the injury and the suppression of EPO production by inflammatory cytokines, just exogenous EPO can be protective against the inflammation.26
NF-κB is a family of DNA-binding protein factors that have an important role for the transcription of pro-inflammatory molecules.27 So, NF-κB can be a basic mediator in the pathogenesis of asthma. The inhibition of NF-κB causes the reduction of allergic lung inflammation and airway hyperresponsiveness.28 NF-κB-binding activity in bronchial mucosa biopsy samples of asthmatic patients was reduced with inhaled budesonide treatment.29 Consequently the inhibition of NF-κB has an important role for the control of pulmonary inflammation.28 In recent studies, it was demonstrated that EPO reduces the production of NF-κB-inducible immune mediators.30 In our study, EPO may reduce the inflammation with the helping of inhibited NF-κB.
The basic mediators of injury are reactive free radicals produced by cellular metabolism under hypoxic condition. Hypoxia leads to synthesis of the hypoxia inducible factor family protein genes, including EPO.26 Cuzzocrea et al. previously demonstrated that EPO reduced arthritis caused by type II collagen in mice, partly by decreasing free radical production.31 EPO significantly reduced the ultrastructural pathological changes and levels of lipid peroxidation in lung tissues after traumatic brain injury. Reactive oxygen species and nitric oxide have been demonstrated to be involved in the pathogenesis of bleomycin-induced pulmonary fibrosis in rodents.14 EPO may therefore also be beneficial against lung injury and fibrosis as an antioxidant agent.
There were some limitations of our study, such as the fact that cytokine levels could not be evaluated and the results found in our study may not translate to positive findings in human clinical trials. Here, to determine the effect of EPO in the treatment of chronic asthma, we evaluated only histopathological changes. Most of the asthma models are devoid of the chronic histopathological changes seen in human asthma because of short term exposure to inhaled antigen. Temelkovski et al. suggested that this experimental model replicated many features of human asthma.12 By the same model, a progressive inflammatory response was developed in the airways of mice characterised by the presence of intraepithelial eosinophils and by infiltration of the lamina propria with lymphoid, mononuclear cells. Goblet cell hyperplasia, epithelial thickening, and subepithelial fibrosis were also evident. Although we could only evaluate the histopathological changes of asthma, the validity of our method increases the value of our study.
In conclusion, the current study has shown that administration of EPO is effective in resolving the established chronic histopathological changes of lungs in a murine model of asthma. Further studies with long-term treatments which evaluate the effects of EPO on lung inflammation and remodelling are needed and especially the cytokine response for the EPO treatment in asthma should be investigated.
Conflict of interestThe authors have no conflict of interest to declare.