Allergic respiratory disease represents a significant and expanding health problem worldwide. The gold standard of therapeutic intervention is still grucocorticosteroids, although they are not effective in all patients and may cause side effects. Allergen Immunotherapy has been administrated as subcutaneous injections for treatment of allergic rhinoconjunctivitis and asthma and has been practiced for the past century. Sublingual immunotherapy (SLIT) tablets are now available for grass- or ragweed-induced rhinoconjunctivitis and will be available in Spain for house dust mite (HDM)-induced rhinoconjunctivitis and asthma in the next months. In this review, new developments in the field of tablet-based SLIT for respiratory allergy are summarized, with special emphasis on HDM-induced allergic rhinitis and asthma. SLIT tablets are the best-documented immunotherapy products on the market and represent a more patient-friendly concept because they can be self-administrated at home.
Sublingual immunotherapy (SLIT) tablets have been developed to treat pollen-induced allergic rhinoconjunctivitis (grass pollen in Europe and ragweed pollen in USA) and house dust mite (HDM)-induced rhinoconjunctivitis and bronchial asthma. Fast dissolving SLIT-tablets have been developed by the industry in clinical programs comprising phase I tolerability studies, phase II dose-finding studies, and large phase III efficacy and safety investigations, including studies in patients with rhinoconjunctivitis as well as asthma. Due, at least in part, to the emergence of these large studies, new European Guidelines for Immunotherapy have been established by European Medicines Agency (EMA). Thus, SLIT tablets are the best-documented immunotherapy products on the market.
In Europe, three products have been developed in accordance with applicable regulatory requirements for marketing authorization. The SQ grass and HDM SLIT tablets (ALK, Horsholm, Denmark) and the 5-grass SLIT tablet (Stallergenes, Antony, France). The SQ grass SLIT tablet (Grazax®, ALK) is a fast-dissolving tablet of a freeze-dried formulation containing grass pollen extract from one grass species, Phleum pretense,1 whereas the 5-grass SLIT tablet (Oralair®, Stallergenes) is a multiparticulate tablet produced by compression and containing a mixture of pollen from five homologous grass species with high IgE cross-reactivity.2
SLIT tablets in allergic rhinoconjunctivitisIn Europe, SLIT tablets have been developed for treatment of grass- or HDM-induced rhinoconjunctivitis. Several “large trials” were conducted in the past 10 years and included dose-ranging studies conducted with standardized extracts of grass and mite.
SLIT tablets for grass pollen-induced rhinoconjunctivitisIn the case of grass pollen allergy two seminal randomized, double-blinded placebo controlled trials led to the approval of grass SLIT tablets in Europe, The first, by Durham et al.3 was a dose-finding study that compared placebo and 0.5, 5 and 15μg of Pl p5 daily. This study found a significant reduction of symptoms and drug consumption vs placebo with the highest dose, which was subsequently accepted as the standard dose.4,5 The other dose-finding study with grass tablets (mixture of 5 grasses) compared placebo and 8, 25 and 42μg/ml of major allergen daily. The dose of 25μg [300 index of reactivity (IR)] showed the best performance in efficacy and side effects6 and was approved by the EMA.
The largest immunotherapy trial performed to date involved 1501 adults and children with grass pollen-induced allergic rhinoconjunctivitis, of whom 85% were polysensitized and 25% had asthma.7 Use of grass pollen talets daily for 20 weeks resulted in a 20% decrease in rhinoconjunctivitis symptoms and a 23% decrease in total combined scores (symptoms plus rescue medication) compared with placebo. Additionally, specific analysis conducted in mono- and poly-sensitized patients demonstrated that, during the grass pollen season, polysensitized patients with allergic rhinoconjunctivitis benefit at least as much, if not more, from the grass pollen tablets as mono-sensitized patients.8,9
Concerning security, local allergic reactions were very common (oral pruritis in 46% and mouth edema in 18%) in the first 1–2 weeks of treatment, but generally disappeared when treatment was continued. Also, these reactions were less common and less severe when treatment was restarted before the next pollen season. Discontinuation due to tablet-related adverse reactions, mostly moderate-severe local reactions in the oral cavity, was very similar for both tablets10 (close to 5%).
On the other hand, it has been demonstrated that clinical benefit of grass pollen SLIT-tablet identified during the treatment period, is maintained for a long-time period; thus, SLIT tablets have a disease-modifying effect. The results of two studies of grass pollen allergen tablet immunotherapy administered daily either pre-coseasonally11 or continuously12 for 3 years were remarkably similar. In both studies, there was an approximate 30–40% reduction in symptoms and rescue medication use during the 3 years period of therapy. Furthermore, the beneficial effect of immunotherapy was maintained for at least 2 years after stopping treatment.
SLIT tablets for HDM-induced rhinoconjunctivitisRecent studies have also demonstrated the efficacy and safety of SLIT tablet in subjects with perennial allergic rhinitis sensitized to HDM. In a randomized, double blind, single-site trial,13 124 adults with HDM-induced allergic rhinitis with or without conjunctivitis and with or without asthma received 12 Developmental Units (DU) of HDM SLIT-tablet (ALK), 6 DU of HDM SLIT-tablet or placebo daily for 24 weeks. Subjects underwent 6-h exposure challenges in an environmental exposure chamber (the Vienna Challenge Chamber) at screening and weeks 8, 16 and 24. The results demonstrated dose- and time-dependent improvements with HDM SLIT-tablet versus placebo. At the end of treatment period, the mean total nasal symptom score (TNSS) improvement relative to placebo was 48.6% with 12 DU and 26.6% with 6 DU. The HDM SLIT-tablet was well tolerated and no investigator-assessed anaphylactic allergic reactions or reactions requiring adrenalin were observed. Therefore, it was suggested that the dose of 12 DU was appropriate for further evaluation to determine the magnitude of effect in a natural allergen exposure environment.
The results of an study published more recently using a different HDM SLIT-tablet (Stallergenes) were consistent with those of the study previously reported.13 Roux and coworkers14 sought to assess the efficacy and safety of 3 doses of HDM SLIT-tablet in an environmental exposure chamber. Adults with HDM-induced allergic rhinitis were given a daily sublingual tablet containing placebo or HDM allergen extract at a dose of 500 IR, 300 IR, or 100 IR for 6 months. Participants registered their rhinitis symptoms during 4-hour HDM environmental exposure chamber challenges at randomization and after 1, 2, 4, and 6 months of treatment. The mean differences from placebo in the area under the curve of the rhinitis total symptom score for the 500 IR, 300 IR and 100 IR groups indicated a dose-dependent effect, with reduction in symptom scores of 33%, 29% and 20%, respectively. The more frequent adverse events were throat irritation and oral pruritus. There were no reports of anaphylaxis, but adverse events leading to premature discontinuation were more common in the 500 IR group. Therefore, the dose of 300 IR was selected for further development of this treatment.
A phase II trial15 with the HDM SLIT-tablet (ALK) showed a beneficial effect on combined rhinitis symptom and medication scores in a subgroup of asthmatic patients with symptomatic allergic rhinitis at inclusion.
The results of two-phase III studies with the HDM SLIT-tablet (ALK) were recently published. In a randomized, double-blind, placebo-controlled trial conducted in 12 European countries,16 a total of 992 adults with moderate-to-severe HDM-induced allergic rhinitis despite treatment with pharmacotherapy received a daily sublingual tablet containing placebo, 6 SQ-HDM or 12 SQ-HDM for approximately 12 months. The results demonstrated absolute reductions in total combined rhinitis score of 1.18 (P=0.002) and 1.22 (P=0.001) compared with placebo for 6 SQ-HDM and 12 SQ-HDM, respectively. The statistically significant treatment effect on this primary end point was evident from 14 weeks of treatment onward. For all key secondary end points, efficacy was confirmed for 12 SQ-HDM, whereas adverse events observed with both doses were similar. Therefore, this study confirms the efficacy and favorable safety profile of both 6 SQ-HDM and 12 SQ-HDM-tablet in adults with HDM-induced allergic rhinitis. These results have been recently replicated in North American adults and adolescents17 with HDM-induced allergic rhinitis. In this group of subjects, treatment with 12 SQ-HDM was associated with an improvement of the total combined rhinitis score of 17% versus placebo.
In spite of the high quality evidence demonstrating that SLIT-tablet induces a significant improvement of symptoms and a reduction in the use of rescue medication for allergic rhinitis, a recent meta-analysis18 concluded that treatment with the grass pollen sublingual tablet was associated with a small reduction in symptoms and use of symptomatic medication (antihistamines and corticosteroids) in patients with seasonal allergic rhinoconjunctivitis. In the opinion of authors of this meta-analysis, given the low magnitude of the benefit, the convenience and easy administration do not seem to be sufficient reasons for the choice of grass-pollen SLIT-tablet. The methodological aspects and interpretation of the results of this meta-analysis have been criticized.19 In particular, the suggestion that the clinical improvement obtained with symptomatic medication is superior to that of SLIT-tablet appears to be incorrect, because recent investigations by Devillier et al.20 concluded that grass pollen SLIT had a greater clinical effect that symptomatic treatments, such as nasal corticosteroids, H1 antihistamines, azelastine-fluticasone propionate combination and montelukast.
More recently, a pooled analysis was performed to indirectly evaluate the effect of SLIT-tablets compared with pharmacotherapy in patients with seasonal or perennial allergic rhinitis.21 Data from 6 timothy grass SLIT-tablet trials (n=3094), 2 ragweed SLIT-tablet trials (n=658) and 2 HDM SLIT-tablet trials (n=1768) were evaluated. Improvements in total nasal symptom scores relative to placebo were compared with those identified in 7 trials with montelukast 10mg (n=6799), 9 trials with desloratadine 5mg (n=4455) and 8 trials with 200μg daily of mometasone furoate nasal spray (n=2140). The results of these analyses indicated that, in patients with seasonal allergic rhinitis, timothy grass and ragweed SLIT-tablets had less effect on nasal symptoms compared with an intranasal corticosteroid, but had a numerically grater effect compared with a leukotriene antagonist and an antihistamine (Table 1). In the group with perennial allergic rhinitis, the effects of HDM SLIT-tablets were numerically greater than all pharmacotherapies. In addition, the effect size with SLIT-tablet was nearly identical for seasonal and perennial allergic rhinitis, whereas the effect size of pharmacotherapies for perennial allergic rhinitis was approximately 1.5- to 2-fold less than for seasonal allergic rhinitis (Table 1). Therefore, although comparisons are limited by study design heterogeneity and use of rescue medications in SLIT-tablet trials, it is evident that, in subjects with allergic rhinitis, the effect of SLIT-tablet is similar to improvement obtained with intranasal corticosteroids, H1 antihistamines and leukotriene receptor antagonists.
Standardized mean differences (SMDs) for SLIT-tablets and pharmacotherapies for seasonal and perennial allergic rhinitis.21
| Treatment | SAR, SMD (95%CI) | PAR, SMO (95%CI) |
|---|---|---|
| Timothy grass SLIT-tablet | −0.26 (−0.33 to −0.19) | |
| Ragweed SLIT-tablet | −0.29 (−0.44 to −0.13) | |
| HDM SLIT-tablet | −0.24 (−0.33 to −0.14) | |
| Montelukast | −0.18 (−0.25 to −0.12) | −0.12 (−0.19 to −0.05) |
| Desloratadine | −0.25 (−0.34 to −0.16) | −0.13 (−0.20 to −0.05) |
| MFNS | −0.61 (−0.74 to −0.48) | −0.28 (−0.40 to −0.17) |
SAR, seasonal allergic rhinitis; PAR, perennial allergic rhinitis; SLIT, sublingual immunotherapy; HDM, house dust mite; MFNS, mometasone furoate nasal spray.
The effect of SLIT-tablet for treatment of allergic bronchial asthma has been investigated more recently. In an initial phase II/III study,22 604 subjects aged more than 14 years with HDM-induced allergic rhinitis and mild-to-moderate asthma were randomized to double-blind treatment with one of 3 active doses (1, 3, or 6 SQ-HDM) or placebo. The use of the inhaled corticosteroid budesonide was adjusted and standardized at baseline, switching the current inhaled corticosteroid to an equivalent dose of budesonide. Then, each patient's dose was reduced to find the lowest dose providing asthma control, with further reduction in steps with intervals of 3–4 weeks until loss of control occurred. In such case, the dose was increased to the previous step to regain control. This dose adjustment process was repeated at the end of the trial, and the primary end point was the reduction in budesonide dose from the individual subject's baseline dose after 1-year of treatment.
The results showed a mean difference between 6 SQ-HDM and placebo in the reduction in daily-inhaled corticosteroid dose of 81μg (P=0.004). This represents a relative mean and median reduction of 42% and 50% for 6 SQ-HDM and 15% and 25% for placebo, respectively. Therefore, in subjects with HDM-induced mild-to-moderate asthma, treatment with HDM SLIT-tablet was associated with a moderate statistically significant reduction in the ICS dose required to maintain asthma control. This was reflected by the following statement in the last version of GINA guideline23: “A study of SLIT for HDM in patients with asthma and HDM allergic rhinitis demonstrated a modest reduction of ICS with high dose SLIT”. Clearly, it is difficult to justify the addition of HDM SLIT-tablet to obtain this small reduction in the dose of inhaled corticosteroid. However, it is evident that these modest reductions in the inhaled corticosteroid doses were, at least in part, consequence of accepting an inhaled corticosteroid dose as low as 100μg/day at inclusion; this leaves little room for improvement in terms of a reduction in the use of inhaled corticosteroids.
In a post hoc analysis of the previously discussed trial, de Blay et al.24 found that subjects with a daily budesonide use of 400–800μg and partially controlled asthma, [defined as an asthma control questionnaire (ACQ) between 1 and 1.5] at randomization, had significantly higher treatment effect (P<0.001) in terms of inhaled corticosteroid dose reduction than the other trial population with less severe asthma. In particular, the 6 SQ-HDM group had significant improvement in asthma control and asthma-related quality of life. In this group, the difference between placebo and active group in change from baseline in daily budesonide use was 327μg.
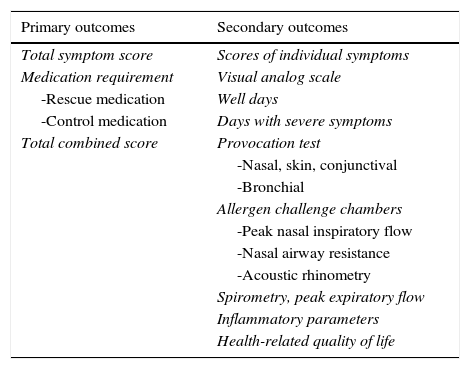
According to recommendations postulated by the EMA,25 the preferred primary outcome measure for allergen immunotherapy clinical trials is the total combined score, which is the sum of the total symptom score and total medication score. The primary and secondary outcome parameters that have been used in clinical trials on allergen immunotherapy have been recently reviewed26 and are reported in Table 2.
Primary and secondary outcomes used in allergen immunotherapy trials.26
| Primary outcomes | Secondary outcomes |
|---|---|
| Total symptom score | Scores of individual symptoms |
| Medication requirement | Visual analog scale |
| -Rescue medication | Well days |
| -Control medication | Days with severe symptoms |
| Total combined score | Provocation test |
| -Nasal, skin, conjunctival | |
| -Bronchial | |
| Allergen challenge chambers | |
| -Peak nasal inspiratory flow | |
| -Nasal airway resistance | |
| -Acoustic rhinometry | |
| Spirometry, peak expiratory flow | |
| Inflammatory parameters | |
| Health-related quality of life |
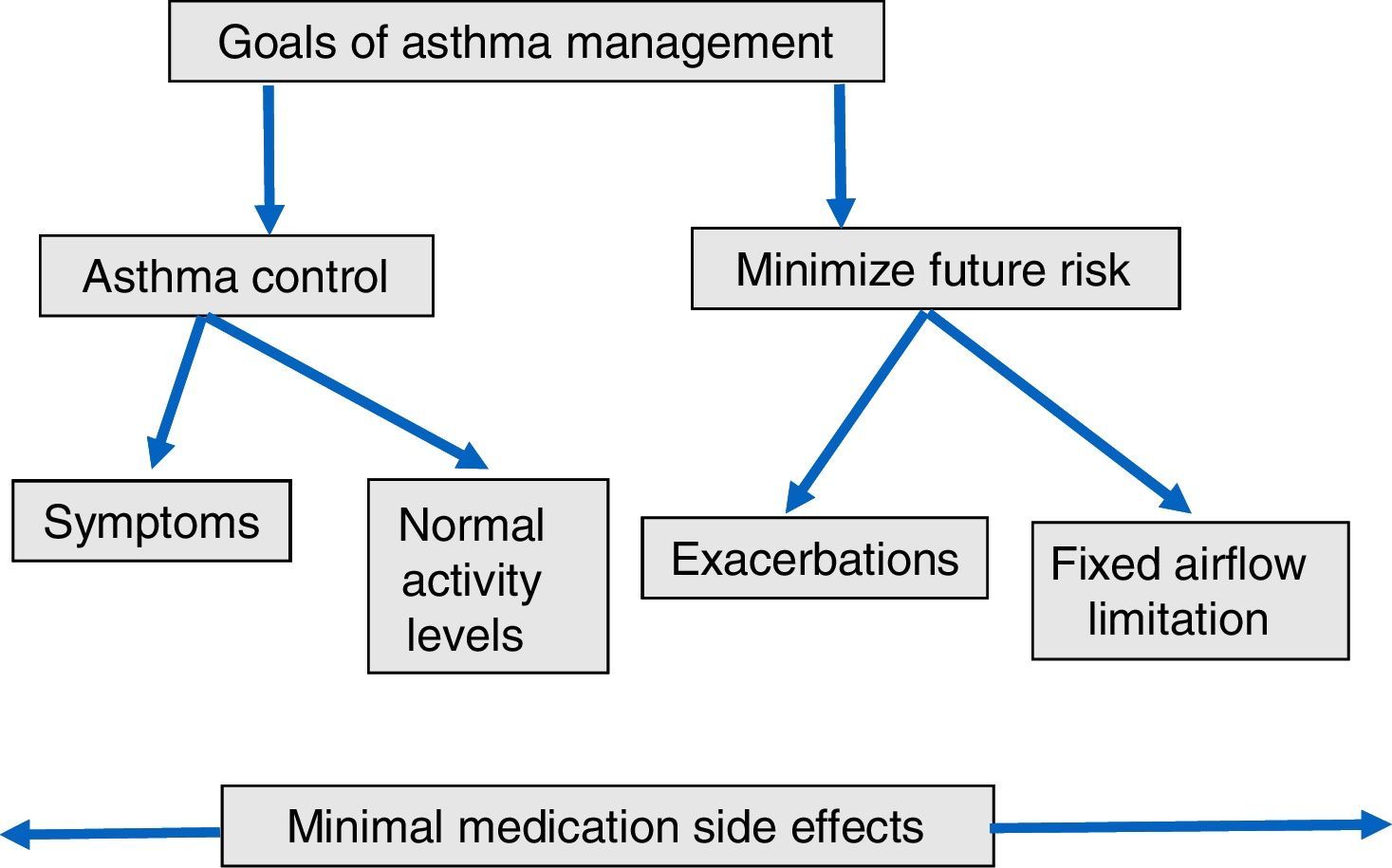
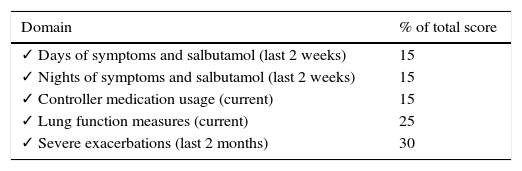
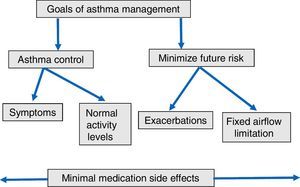
By contrast, GINA guideline23 recommends the following long-term goals of asthma management (Fig. 1): (1) to achieve good control of symptoms and maintain normal activity levels; and (2) to minimize future risk of exacerbations, fixed airflow limitation and side-effects. According with these suggestions, new instruments have been developed to evaluate the multidimensional nature of asthma control and to determine the effect of allergen immunotherapy on asthma exacerbations as a primary outcome. The Composite Asthma Severity Index (CASI)27 was designed to combine the proposed facets of asthma control. In this index, the maximal weight of the dimensions of asthma severity was assigned to exacerbations (Table 3). Instruments such as CASI that go beyond simply measuring asthma control are necessary to assess the effectiveness of immunotherapy in the context of guidelines-based care. More recently, the conceptual definition for moderate asthma exacerbation provided by the ATS/ERS consensus statement of terminology28 has been adopted for use in clinical research. Criteria for identify a moderate asthma exacerbation are indicated in Table 4.
Clinical weighting of each domain in the composite asthma severity index (CASI).27
| Domain | % of total score |
|---|---|
| ✓ Days of symptoms and salbutamol (last 2 weeks) | 15 |
| ✓ Nights of symptoms and salbutamol (last 2 weeks) | 15 |
| ✓ Controller medication usage (current) | 15 |
| ✓ Lung function measures (current) | 25 |
| ✓ Severe exacerbations (last 2 months) | 30 |
Defining moderate asthma exacerbation.28
| (a) Nocturnal awakening(s) due to asthma requiring SABA for 2 consecutive nights or increase of ≥0.75 from baseline in daily symptom score on 2 consecutive days |
| (b) Increase from baseline in occasions of SABA use on 2 consecutive days (minimum increase: 4 puffs/day) |
| (c) ≥20% decrease in PEF same from baseline on at least 2 consecutive mornings/evenings or ≥20% decrease in FEV1 from baseline |
| (d) Visit to the emergency room/trial site for asthma treatment nor requiring systemic corticosteroids |
A moderate exacerbation is defined as ≥1 of criteria (a)–(d) fulfilled and leading to a change in treatment.
According with these previous suggestion on primary outcomes in asthma treatment, the big phase III study on the efficacy of HDM SLIT-tablets in asthma was designed with asthma exacerbations as a primary outcome. Virchow and colleagues29 conducted a double-blind, randomized, placebo-controlled study that included 834 subjects with HDM-induced allergic asthma not well controlled by inhaled corticosteroid or combination products. Patients with severe or unstable asthma, defined as a FEV1 less than 70% of predicted value or hospitalization within the previous 3 months, were excluded. Participants were randomized to once-daily treatment with placebo (n=277) or HDM SLIT-tablet [dosage groups: 6 SQ-HDM (n=275) or 12 SQ-HDM (n=282)] in addition to inhaled corticosteroids and the short acting beta2-agonist salbutamol. Efficacy was assessed during the last 6 months of treatment when inhaled corticosteroid doses were reduced by 50% for 3 months and then completely withdrawn for 3 months.
The primary outcome, defined as the time to the first moderate or severe asthma exacerbation during the inhaled corticosteroid reduction, was improved by both doses of active SLIT-tablet compared with placebo, with an absolute risk for first exacerbation of 26% for the 6 SQ-HDM group, 24% for the 12 SQ-HDM group, and 32% for the placebo group. No significant differences were identified between the 2 active treatment groups. The most frequent adverse event was mild-to-moderate oral pruritus (13% for the 6 SQ-HDM group, 20% for the 12 SQ-HDM group, and 3% for the placebo group), but systemic or severe allergic reactions were not reported. The study represents a relevant contribution to the literature on the efficacy of allergen immunotherapy in asthma, mostly because the selection of a highly relevant primary end point, such as time to the first asthma exacerbation. The goals of future studies on allergen immunotherapy in asthma should be to identify ideal candidates to each immunotherapy regimen, using clinical or physiological characteristics or specific biomarkers.
Conflict of interestL. Prieto reports grants and personal fees from Novartis, ALK Abello, Stallergenes, Teva, GSK.