Exposure to fungi has two main features: in addition to its allergenicity, it is a major risk factor for individuals with a predisposition to allergic disease. Exposure remains predominantly external but there are increasing references to indoor exposures.1–5 The prevalence of sensitivity to these sources of sensitization is not clear. The dispersion of data due to the use of extracts with different allergenic profiles,6 caused by different levels of expression of the strain selected,7–10 the conditions and characteristics of cultivation and growth, and the selection of the most appropriate protocol for the extraction of the proteins expressed, result in wide variability in epidemiological data.11–19
There are an estimated 1–1.5 million fungal species worldwide, of which around 80,000 have been described: only 112 are considered allergenic sources.20 The four genera most commonly associated with allergic disease are Alternaria,21Cladosporium, Penicillium and Aspergillus. A total of 107 allergens from 28 fungal genera have been accepted by the International Union of Immunological Societies (IUIS) subcommittee that lists allergens internationally, and form part of its database.
Fungi are known to produce a wide range of reactive IgE responses, and the factor that determines this variability is the recognition of the proteins that constitute an allergenic source. The definition of major allergen (one that originates specific IgE responses in more than 50% of patients sensitized to the allergen)22 has a local connotation and its binding capacity with the IgE from a specific allergic patient does not always reflect its clinical relevance. In the case of the genus Alternaria and, specifically, Alternaria alternata species, the major allergen Alt a 1 is recognized by more than 90% of individuals sensitized to this source globally and by a large percentage of asthmatic patients. Alt a 1 is present in more than 50 phylogenetically-related species,20,23 and may be considered as the allergen that best defines the diagnosis of allergy to fungi in our setting, and as a replacement for the complex and heterogeneous extract of Alternaria alternata as a diagnostic and therapeutic tool in respiratory allergy caused by fungi.
The International Council for the harmonization of technical requirements for medicinal products for human use (ICH) proposes the use of a guideline or common technical document (CTD)24 in order to achieve greater equality in the technical characteristics of candidate drugs for marketing authorization to ensure the safety and efficacy of the product used in the diagnosis and treatment of allergic patients.
In this sense, the technical characteristics of the major allergen Alt a 1 purified from an extract of Alternaria alternata are ordered according to the recommendations of the aforementioned guide and based on the criteria recommended in it.
The chapter on technical criteria is based on the description of the process of obtaining the active substance (purified Alt a 1) by validated chromatographic methods, the subsequent analysis of the physicochemical and immunological characteristics of the substance, and the configuration of the final product based on criteria of biological activity, stability and sterility, and on the absence of toxic and irritating elements, in addition to those already mentioned in the chapter on the active substance.
The biological activity of Alt a 1 was evaluated according to the Nordic guidelines, and applying their methodology and criteria. This showed that 1 HEP (10,000UB/mL) corresponded to 5.97g/mL of Alt a 1, with a 95% confidence interval ranging from 1.71 to 7.16μg/mL.
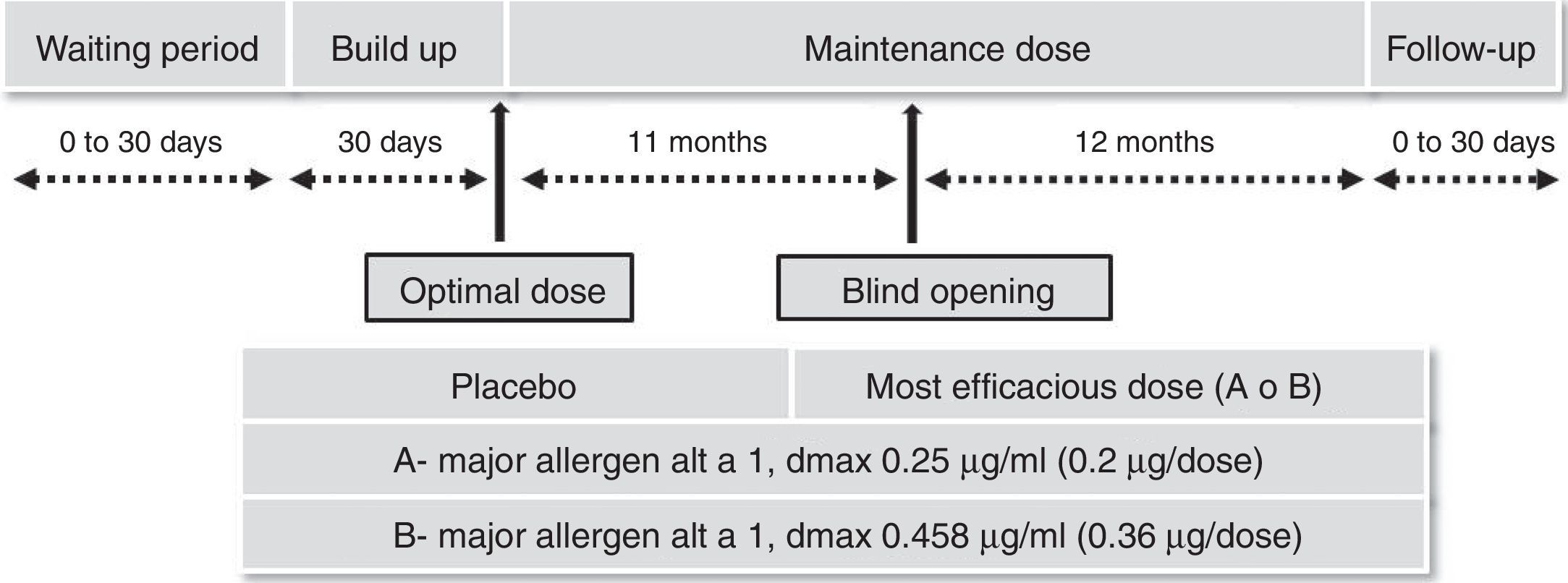
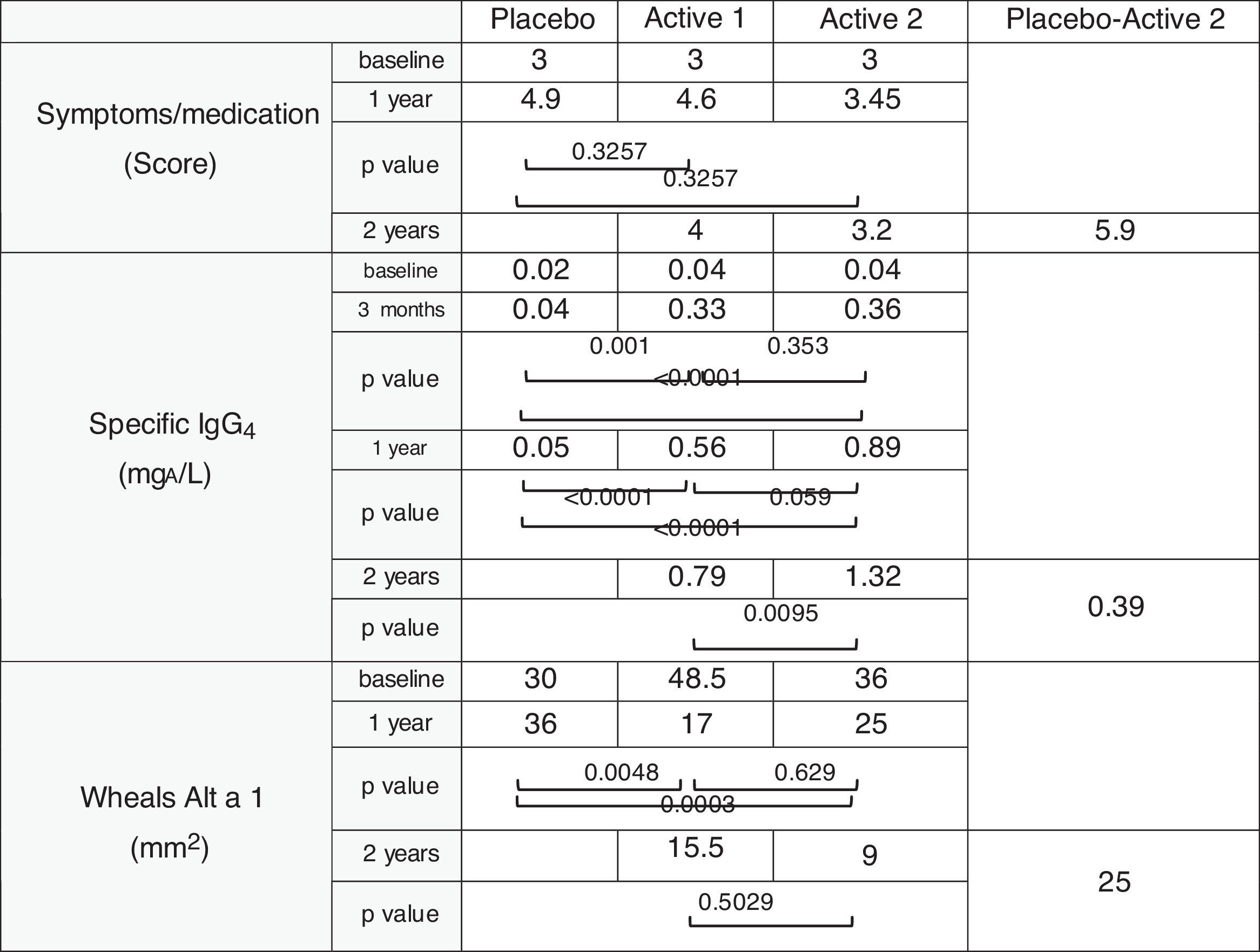
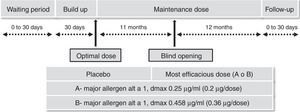
Safety and efficacy trials25 were made using a randomized, double-blind, placebo-controlled design in patients with allergic rhinitis with or without mild-to-moderate asthma, as indicated by the EMA guidelines on immunotherapy with allergenic extracts. Significant increases in immunogenicity and reductions in the combined symptoms/medication index and cutaneous reactivity.
The most effective and safest dose was 0.36mg of Alt a 1 given monthly for two years.
Fig. 1 shows the segregation by groups and the different stages of the trial and Table 1 the results obtained.
The author reports being employed by Diater Laboratories, a pharmaceutical company specializing in the production of allergenic extracts for in vivo diagnosis and treatment of allergies.
No other potential conflict of interest relevant to this letter was reported.