Th17 lymphocytes are now widely believed to be critical in various chronic pulmonary diseases. However, there is still a small number of investigations regarding children. We aimed to assess the percentage of Th17 lymphocytes and IL-17A in peripheral blood of children with chronic obstructive lung diseases.
Patients and methodsWe included a total of 42 children: 20 with bronchial asthma (BA), 12 with cystic fibrosis (CF) and 10 healthy children without a history of allergies, aged 4–17 years. Th17 cells (CD3+CD4+CD161+CCR6+) were determined in peripheral blood by flow cytometry. The concentration of serum IL-17A was measured by ELISA.
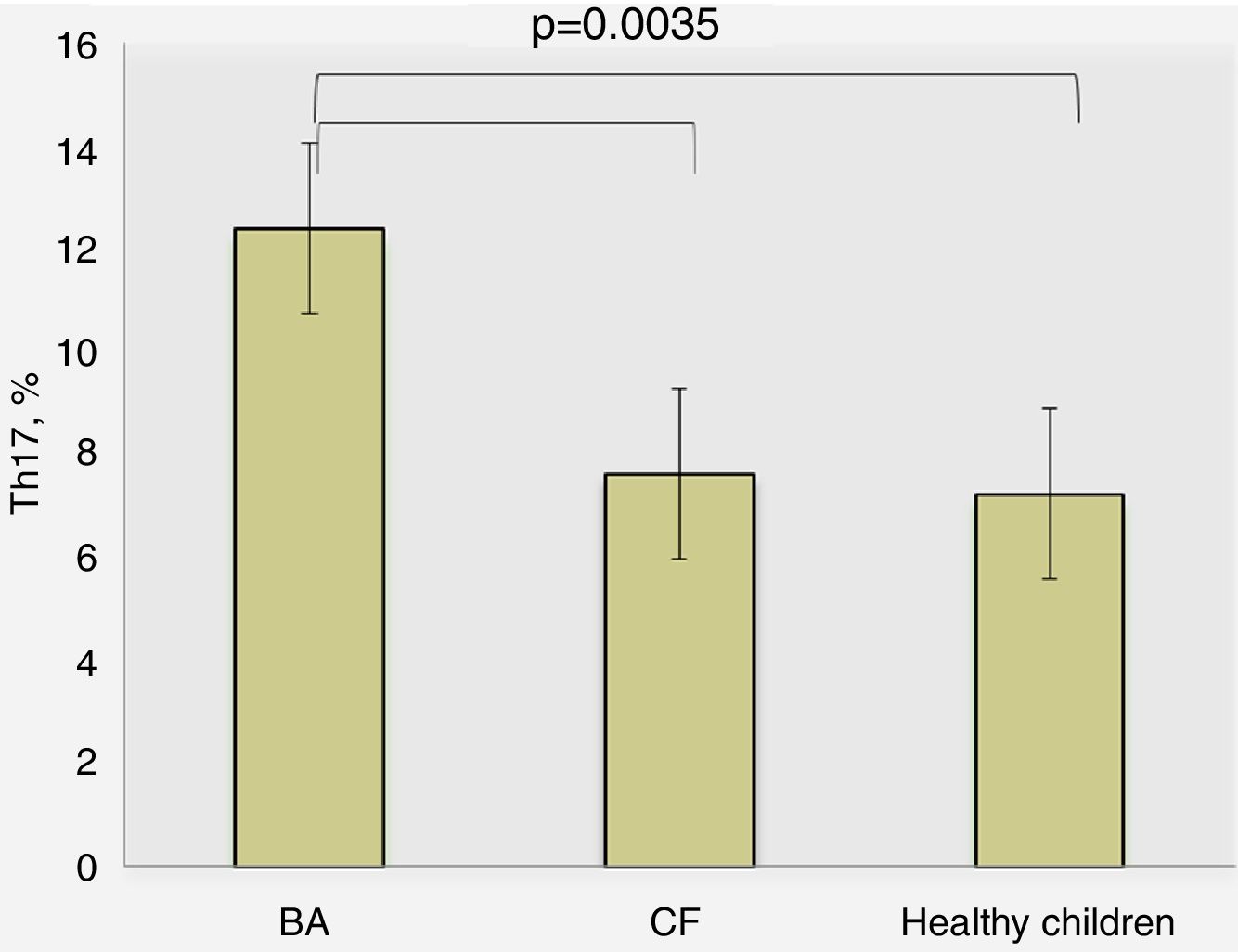
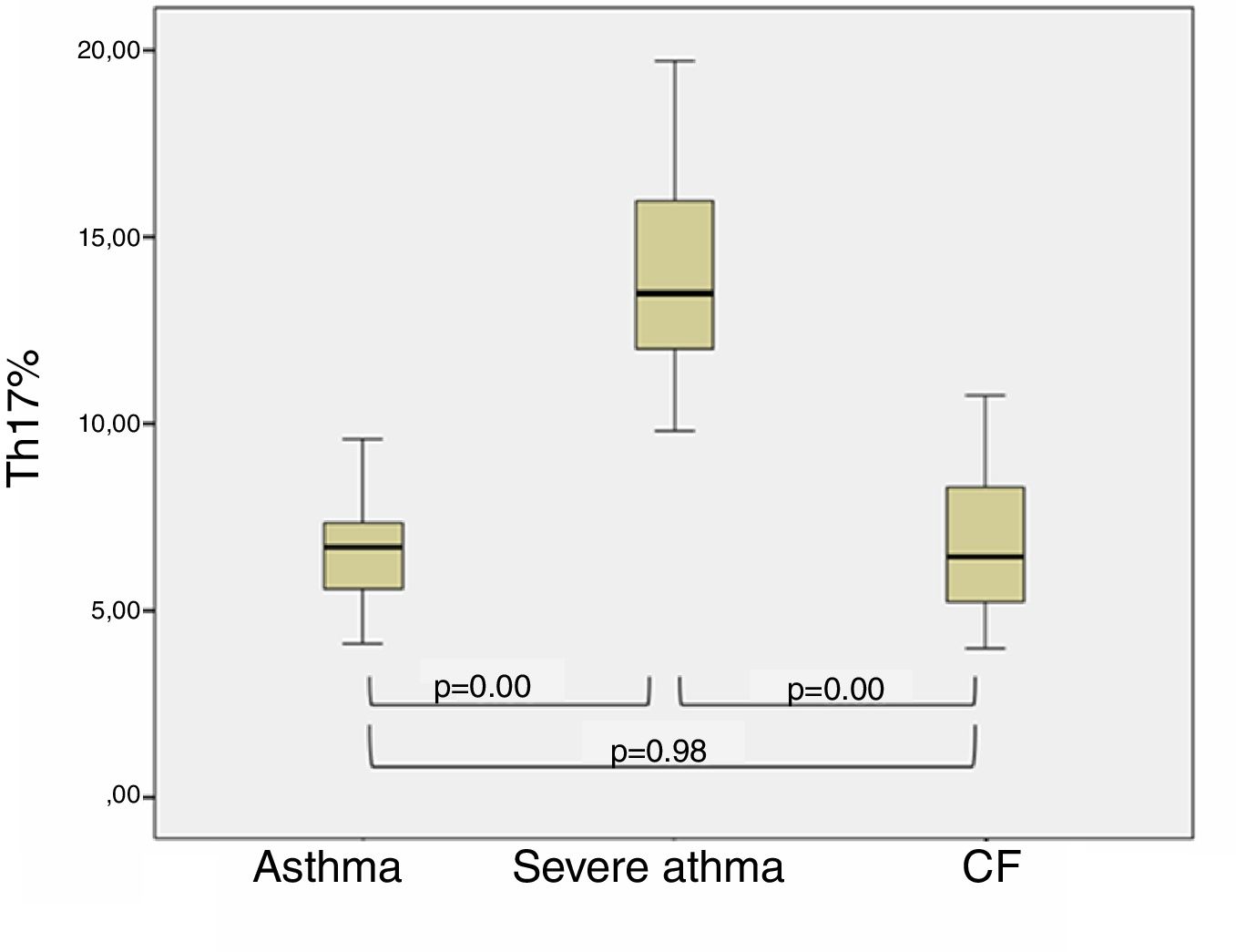
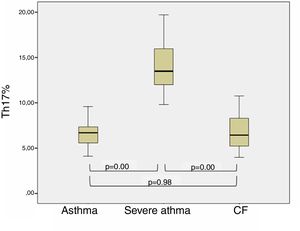
ResultsThe BA patients had a significantly higher percentage of Th17 (12.40±1.16%) compared to the CF children (7.64±0.87%, p=0.0035) and healthy (7.25±0.45%, p=0.008). Stratifying the BA group, we found higher levels of Th17 in patients with severe BA (p=0.03), whereas patients with moderate BA had Th17 cells close to those in CF and healthy children. We found that patients with better control of BA had Th17 closer to those with CF (p=0.98) than BA children with poor control (p<0.001) (post hoc, Bonferroni correction). CF patients with concomitant P. aeruginosa infection showed slightly higher percentages of Th17 cells than those without infection (8.08±3.09% vs. 6.25±2.42%, p=0.294).
ConclusionsThe percentage of Th17 cells was significantly increased in the peripheral blood of children with severe BA compared to the children with moderate BA, which suggests that the former could possibly benefit from future target therapies.
Bronchial asthma (BA) is a complex and heterogeneous disease characterized by intermittent and reversible obstruction, chronic airway inflammation, bronchial hyperreactivity and infiltration of respiratory submucosa with immune cells.1 Over the past decades, a large number of epidemiological studies have found an increase in the incidence of BA, mainly due to the rise in childhood asthma. It is one of the most common chronic diseases in childhood affecting 8–10% of all children. The chronic airway inflammation recruits many cell types, including T-lymphocytes, eosinophils, mast cells, macrophages, epithelial cells, fibroblasts and bronchial smooth muscle cells,2 which release pro-inflammatory cytokines and cytotoxic mediators. This results in variable, diffuse, reversible obstruction as well as hyper bronchial response to various specific and non-specific stimuli. In about 80% of cases, BA is diagnosed before the age of six. The natural course of the disease is characterized with a progressive decline in respiratory function (i.e. forced expiratory volume for 1 second, FEV1) and persistent bronchial hyperreactivity, which are accompanied by both inflammation and structural remodeling of the airways.2
Advances in the study of the pathogenesis in recent years have led to the identification of different groups of asthma – phenotypes and endotypes.3,4 Moreover, suitable biomarkers for diagnosis and follow-up of the disease could be revealed by exploring the various underlying mechanisms.5 Since the different types of asthma are driven by disparate pathophysiological processes, the expectation is that the target therapies will be applied to the respective patient groups.4
At present, BA is thought to be an immune-mediated disease involving T lymphocytes, mast cells, basophils, IgE-producing plasmids and a broad range of cytokines (IL-6, IL-8, IL-12, IL-10, IL-13, IFN-γ, and IL-17).6 IL-17-mediated inflammation is characterized by pathogen or allergen irritation and subsequent differentiation of naive T lymphocytes into IL-17 producing cells. Th17 lymphocytes secrete various cytokines, such as IL-17A, IL-17F, and IL-22, but also induce mucosal and innate immune cells to emit a large number of inflammatory cytokines and chemokines that locally attract more mast cells, eosinophils, and basophils. There is also evidence of the involvement of Th17 subpopulation and their closely related IL-17A and IL-17F cytokines in the induction of mucin production, airway smooth muscle hyperreactivity and corticosteroid-resistant inflammation in mouse models.7–10
It is noteworthy that, despite intense scientific work on the role of Th17 in various diseases, and especially in some respiratory (i.e. BA), studies in childhood are limited. This is understandable due to the difficulty in assessing children clinically along with other limitations. Thus, any research would complement the European and global database. One could suggest that locally enhanced IL-17 immune responses would be associated with elevated circulating Th17 cells, which can be detected in peripheral blood. On this background, we aimed to assess the percentage of Th17 lymphocytes along with IL-17 concentrations in peripheral blood in a cross-sectional study of 42 Bulgarian children with BA, and in control groups of pediatric patients with cystic fibrosis (CF) and healthy ones.
Material and methodsSubjects of the studyThe study comprises 42 children divided into the following groups: 20 with BA, 12 with CF and 10 healthy children at mean age 10±3 years (4–17 years old). The demographic characteristics of the children recruited in the study are presented in Table 1. There was no significant difference in gender distribution (p=0.447) or age (p=0.27) in all groups.
Demographic characteristics of the study groups.
| Diagnosis | Number (%) | Females, number (%) | Males, number (%) | Age, years |
|---|---|---|---|---|
| Bronchial asthma | 20 (47.6) | 7 (36.8) | 12 (63.2) | 11±3 |
| Cystic fibrosis | 12 (28.6) | 6 (50) | 6 (50) | 9±3 |
| Healthy children | 10 (23.8) | 5 (50) | 5 (50) | 9±2 |
The data are presented as mean±SD or number (%).
Clinical, laboratory and instrumental methods were performed in selecting the three groups of children. The appropriate inclusion and exclusion criteria were as follows. We included children after signed informed consent from parents, with the consent of the child to participate in the study; with confirmed diagnosis of BA, or genetically proven CF, or clinically healthy children (with no proven disease including but not limited to Crohn's disease, ulcerative colitis, celiac disease, atopic dermatitis, rheumatoid arthritis, other systemic or psychiatric diseases). The exclusion criteria were: the refusal of parents for their child to participate in the study, refusal of the child to participate, and the presence of unexplained chronic lung disease.
All subjects of the study were informed about the purpose of the experiment, and a written informed consent was obtained from parents/legal guardians. The study design was approved by the Ethical Committee of the Medical University of Sofia, and the research was performed according to the local hospitals’ ethical considerations.
Clinical characteristics of the study groupsThe diagnosis of BA children was made according to the GINA guidelines including the disease, personal atopic and family history and positive bronchodilation test. None of the children was with newly diagnosed asthma, all of them had a physician-confirmed diagnosis for more than 24 months. From the BA patients, ten were with severe BA (characterized by sustained symptoms despite treatment with high doses of intravenous corticosteroids) and ten with moderate BA. All children were followed in the clinic for more than two years and their asthma severity was defined based on symptom frequency and spirometric results (forced expiratory volume in 1 second [FEV1]) and not in one-time point evaluation.11 For all children, we performed pulmonary function tests (PFT) (pre- and post-bronchodilator spirometry), nasal smears for eosinophil counts, we drew blood for IgE against inhaling and food allergens detection, and ACQ (Asthma Control Questionnaire), validated Bulgarian translation and ACQ interviewer-administered version for the age 6–10 years.12
The diagnose of CF was based on clinical presentation, positive sweat tests and confirmed two disease-causing mutations in CFTR gene. All patients were followed in the clinic since the diagnosis was confirmed and were seen regularly for physical examination, pulmonary function tests, therapy evaluation, microbiology samples. Specifically, for the nature of the study, we excluded all children with a history of allergy or elevated eosinophil counts in sputum, and also all the patients that can be defined as CF-asthma.13,14 We evaluated the presence of Pseudomonas aeruginosa in the sputum/throat samples at the time of the study, but also all previous microbiological findings. P. aeruginosa was isolated from their sputum/throat swab samples and also confirmed with antibody testing for proper classification, according to previously described methods.15 From the 12 children with CF included, 50% covered the criteria for chronic P. aeruginosa infection (at least three positive samples in the last two years and/or very high antibody titer) and the remaining six children did not. Some patients had P. aeruginosa in the sputum at the time of the evaluation, but it was their first isolation or they had a very low antibody titer – they were not chronically infected. Other CF patients had previously isolated P. aeruginosa multiple times, but not in the time of Th17 evaluation, but had very high titer – those patients covered the definition for chronically infected.16
Specimen collectionAfter informed consent, venous blood was collected from each subject during a routinely performed blood withdrawal. Peripheral whole blood (EDTA, 1ml) was collected and immediately examined with a specific flow cytometry protocol for Th17 cells labeling and analyzing.17 Serum samples (1–3ml) from each subject were also collected using serum separator tubes and frozen at −80°C before testing for IL-17A.
Flow cytometry analysis of Th17 lymphocytesAnalyses of Th17 cells were performed with Lyse-Wash Protocol after staining the whole blood with the following fluorescence-labeled antibodies: anti-CD3 (FITC), anti-CD (PerCP), anti-CD161 (PE) and anti-CCR6 (AlexaFluor 647) (BD Biosciences, USA). Th17 cells were analyzed by BD FACSCalibur flow cytometer. Up to 10,000 lymphocytes were counted and analyzed using the Cell Quest software program (BD Biosciences, USA). The lymphocytes were gated according to their size and granularity (Forward scatter, FSC, and side scatter, SSC); afterwards CD3+CD4+(T helper) cells were gated; from them, those cells double positive for surface expression of CD161 and CD196 were defined as Th17 cells. Th17 lymphocyte subpopulation determined based on the simultaneous surface expression of CD3, CD4, CD161, CCR6 was adapted from Kerzel et al.,17 as previously described.18 The assay was performed at the Laboratory of Clinical Immunology, University Hospital St. Ivan Rilski, Sofia, Bulgaria.
Immunoenzyme assay for evaluation of IL-17A concentrationsFor the determination of IL-17A in serum samples we used the Human IL-17A ELISA kit (Gene probe, Diaclone, France). According to the manufacturer, the analytical sensitivity of the IL-17 detection assay is 2.3pg/ml. The test was carried out at the Laboratory of Clinical Immunology, University Hospital St. Ivan Rilski, Sofia, Bulgaria.
Statistical analysisThe raw data was analyzed statistically using descriptive statistics, parametric (ANOVA analysis) (i.e. Th17 lymphocytes in the three different groups of children) and non-parametric tests for unrelated samples due to the non-normal distribution of the parameters (Mann–Whitney test), correlation, and post hoc analysis (Bonferroni correction) when an overall statistically significant difference in groups was found, to emphasize which specific groups differed. Statistical analysis was performed with the software package for statistical analysis (SPSS®, IBM 2009, version 19). We accepted the results for significant if p<0.05.
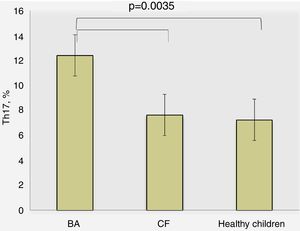
ResultsTh17 lymphocytes and IL-17 in different study groups of childrenDetermination of percentage of Th17 lymphocytes in peripheral blood by flow cytometry revealed the following results: for BA patients 10.40±1.16%, CF – 7.64±0.87%, and healthy children – 7.25±0.45%, where the differences were significant between BA and other study groups (p=0.0035, ANOVA) (Fig. 1). There was no significant difference between the percentage of Th17 lymphocytes in children with CF and healthy children (p>0.05).
The levels of IL-17A were observed as follows: 2.64±2.34pg/ml in BA patients, 2.63±0.19pg/ml in CF patients and 2.67±0.66pg/ml in healthy children. However, the differences were not estimated as significant. We also did not find a significant correlation between the levels of Th17 in peripheral blood and the corresponding concentration of IL-17 in serum.
Th17 lymphocytes and clinical characteristics of children with bronchial asthmaWhen dividing BA patients according to the control and clinical course of the disease in two subgroups, it was found that patients with better control of asthma (no exacerbations during the last year) had percentages of Th17 cells closer to children with CF (p=0.98), in contrast to the children with poor-controlled (severe) asthma (p<0.001, post hoc, Bonferroni correction). The results are presented in Fig. 2.
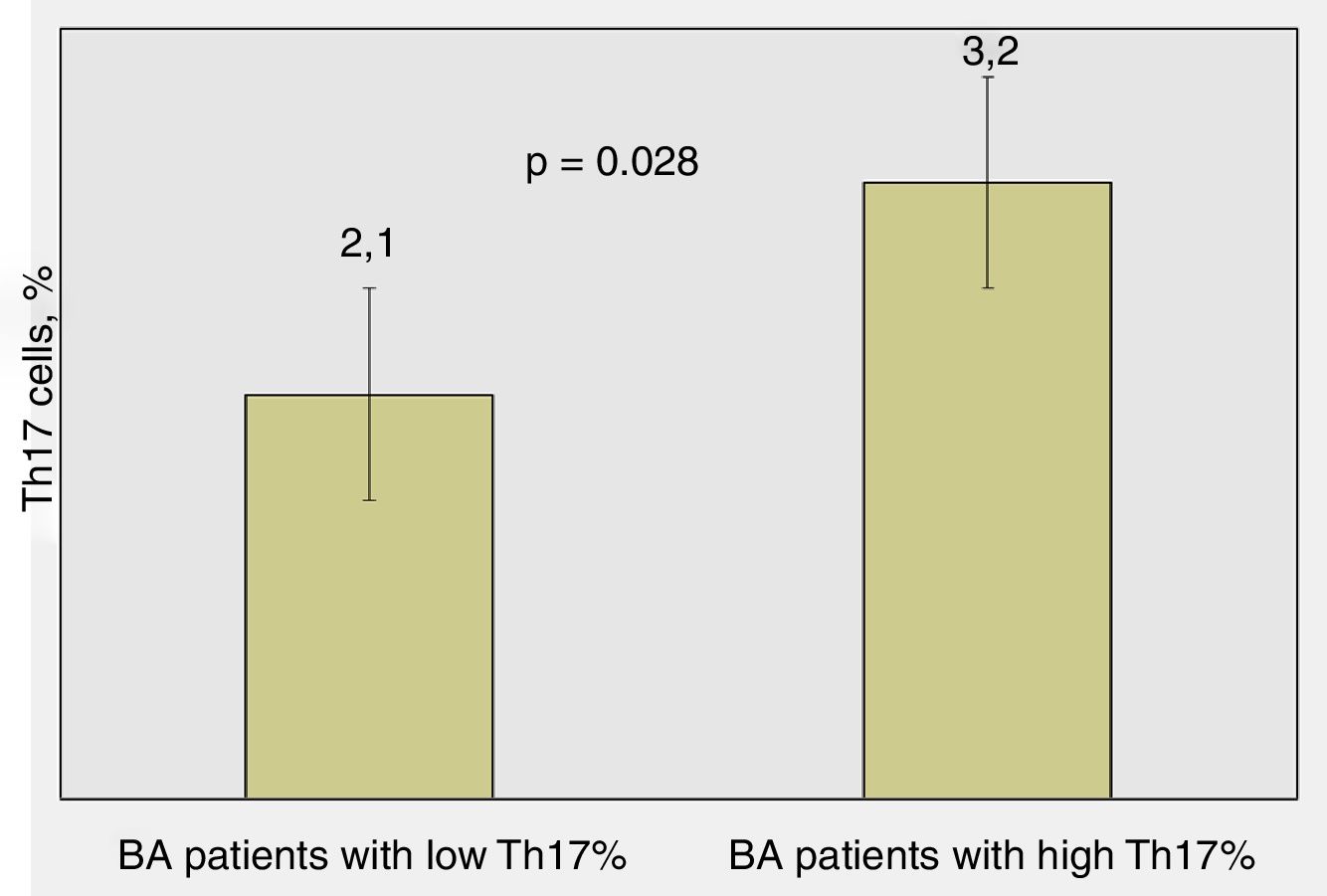
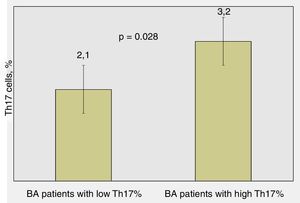
Significant differences were found regarding the number of attacks over the last 12 months in BA children: patients with lower percentages of Th17 cells had average 2.1±1.5 attacks/yearly compared to those with higher percentages of Th17 cells – 3.2±2.1 attacks/yearly (p=0.028, Mann–Whitney test) (Fig. 3).
According to other clinical and laboratory findings in BA children, we found that the higher percentage of Th17 lymphocytes was associated with higher percentage of nasal eosinophils in non-linear dependence. In BA children with poor control (eight of whom with allergic rhinitis), the percentage of Th17 cells were higher than those with good control (five of them with allergic rhinitis) (8.45±1.34% vs. 4.32±1.33%, p<0.05). BA children with lower percentages of Th17 cells performed better at lung function tests compared to children with higher percentages of Th17 lymphocytes (FEV1 96.75±20.38% and a positive bronchodilator response in five out of 11 children vs. FEV1 90.84±20.55% and a positive bronchodilator response in eight out of nine children, respectively, p=0.034).
We did not find a relationship between Th17 levels and the age of BA onset, the duration of the disease; concomitant control therapy.
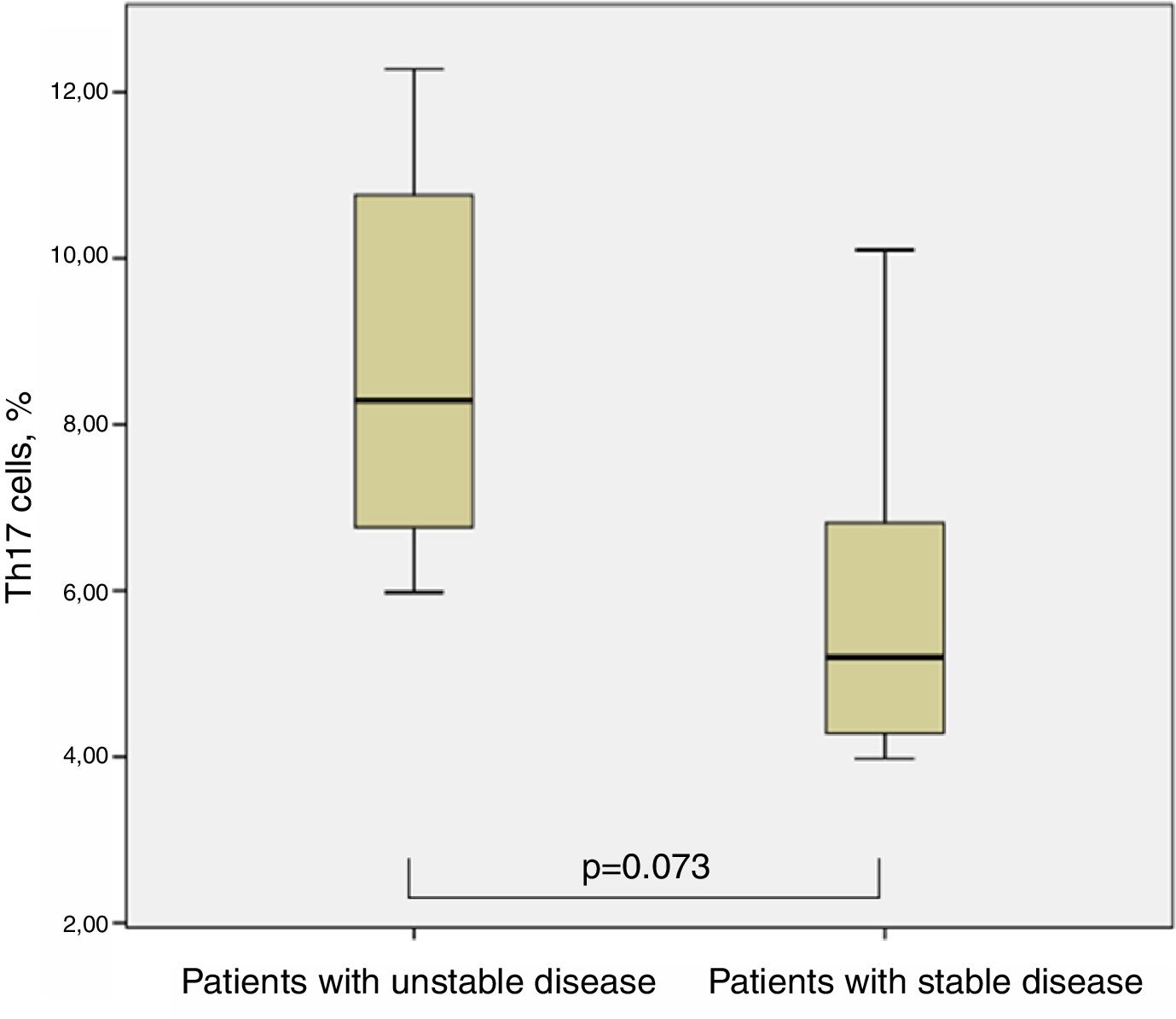
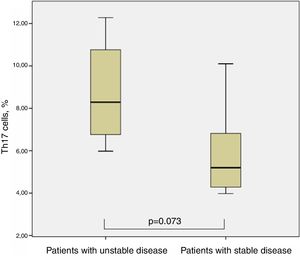
Th17 lymphocytes and clinical characteristics of children with cystic fibrosisIn the case of CF, when we divided them into two groups concerning the isolation of P. aeruginosa at the time of Th17 evaluation, we could not find significant differences between both groups, although those with the presence of P. aeruginosa showed slightly higher percentages of Th17 cells (8.08±3.09% vs. 6.25±2.42%, p=0.294, Man–Whitney test). Seven out of 12 patients required at least one admission to hospital for pulmonary exacerbation in the year before Th17 evaluation (one of them even had four admissions); the other five were in a stable clinical condition and had not required inpatient treatment for more than two years before evaluation. There was a tendency for higher percentages of Th17 cells in the group with less stable condition 8.81±2.66 vs. 5.92±2.37 (p=0.073, Man–Whitney test) (Fig. 4).
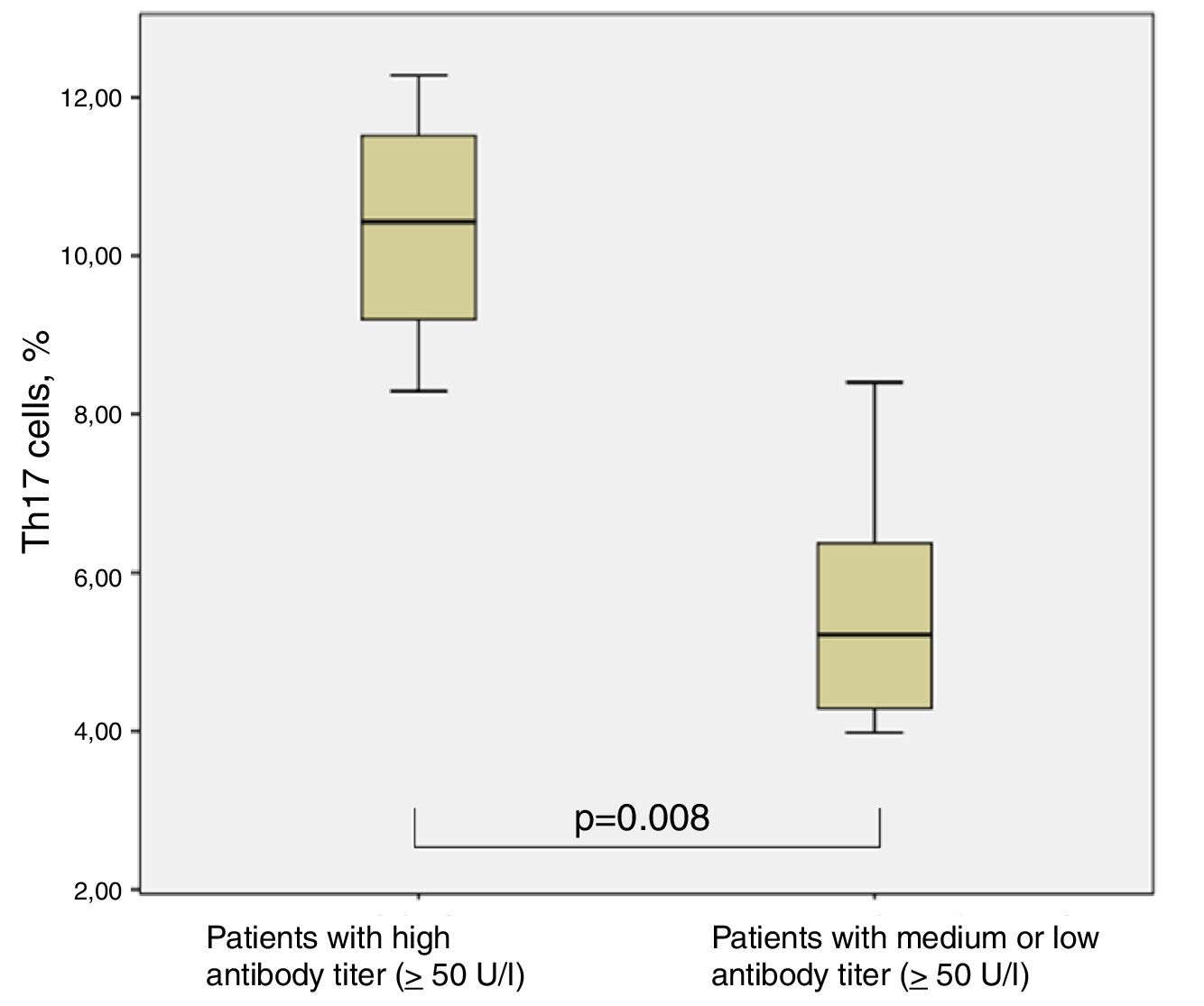
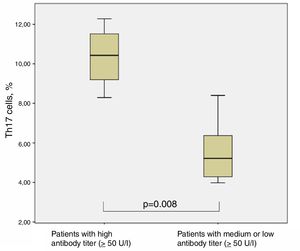
The chronically infected CF patients with very high antibody titer against P. aeruginosa (four out of 12) showed significantly higher levels of Th17 cells (10.35±1.65%) in comparison with all other CF patients (with medium and low antibody titer) (5.51±1.49%) (p=0.008, Man–Whitney test) (Fig. 5).
DiscussionThe results we obtained concerning the percentage of Th17 lymphocytes in the different groups of children with chronic obstructive lung disease are a theoretical contribution in the field of childhood asthma. We found that the percentage of Th17 lymphocytes was significantly higher in children with BA than in children with CF and healthy children. These results are in consensus with the results of Kerzel et al. (2012), which have a close to our research design.17 They also found higher percentages of Th17 lymphocytes in children with BA than in healthy subjects. However, their study did not have a control group of children with CF, as in our study. In this way, our study contributes theoretically for both Bulgarian pediatric science and worldwide.
Moreover, after separation of the group of children with BA according to the control and the clinical course of the disease, we found significantly higher percentages of Th17 cells in patients with poor control and frequent exacerbations which required hospital treatment compared to children with good control (Figs. 3 and 4), which is a valuable contribution with a practical focus. In this way, we could conclude that patients with poorly controlled BA, where Th17 cell counts are significantly elevated, would be candidates for targeted therapy. Studies by Irvin et al. (2014) support the hypothesis that patients with simultaneous presence of Th2 and Th17 lymphocytes are more challenging to treat and have a more severe respiratory obstruction and hyperreactivity.19 There is evidence that an increased level of IL-17 is associated with exacerbation of asthma, with a reduced response to therapy, accumulation of granulocytes, production of fibrotic mediators and remodeling of the airways with marked eosinophilia.7,19 However, we did not document such observations for IL-17 serum concentrations.
The exact mechanisms of Th17 cells involvement in the pathogenesis of BA have not been fully elucidated. It is thought that Th17/IL-23 axis is involved in increased Th2 mediated eosinophilic inflammation and hyperreactivity.7,20–22 In line with this, upregulated gene and protein expression of IL-17 in the lungs has been reported in broncho-alveolar lavage, serum, sputum.19 Furthermore, it was also shown that the severity of bronchial hyperreactivity correlates with IL-17 levels,19 whereas IL-17 in sputum – with an increased response to methacholine.23 Some authors indicate that the elevated level of IL-17 is associated with asthma exacerbation, reduced response to therapy, granulocyte accumulation, fibrotic mediator production and respiratory remodeling, as well as marked eosinophilia.24–26 Moreover, some studies suggested increased serum IL-17 as a marker and independent risk factor for severe asthma.27 In our study, we obtained similar data but for Th17 cells, not IL-17A. We did not detect significant differences in serum IL-17 concentrations in the different groups of children. This may be because the level of IL-17 in serum does not always directly reflect the function and secretion of peripheral blood Th17 lymphocytes. As already mentioned above, there was no established correlation between the percentage of Th17 lymphocytes and IL-17.
Surprisingly, in murine models of asthma, it has also been found that IL-17 may be a negative regulator for the onset of asthma. It was found that endogenous IL-17 is under the control of IL-4.28 Although IL-17 is involved in antigen sensitization and asthma, in sensitized mice IL-17 reduces allergic responses by inhibiting antigen-presenting cells and chemokine synthesis.28 One explanation for this paradox is the participation of the suppressive and tolerogenic T regulatory cells (Treg). However, Treg cells in the conditions of active inflammation and presence of IL-23 promote the development of Th17 cells and the synthesis of IL-17A.29 The produced IL-17 directly inhibits the IL-4, IL-13 and IL-5 cytokines in the lungs and regional lymph nodes, as well as antigen-presenting cells and chemoattractants, thus, reducing allergic responses.30
Previous studies showed that over 90% of children with BA and poor asthma control had accompanying allergic rhinitis and correspondingly nasal eosinophilia, as well as lower respiratory functional performance and strong positive broncho-dilatation test.31 High levels of IL-17 producing CD3+CD4+ cells in peripheral blood of polysensitized allergic rhinitis patients were demonstrated recently in Bulgarian patients.32 Nasal eosinophilia is directly associated with concomitant allergic rhinitis, which may worsen the control of the underlying disease. The small number of children with poor control without allergic rhinitis (one) in the current study did not allow thorough statistical analysis.
According to CF, one of the proposed models for the Th17 cells involvement in pulmonary inflammation is associated with the stimulation by pathogens. P. aeruginosa induces synthesis of IL-6 and IL-23. Together with TGFb1, the cytokine milieu pushes the differentiation of naive T cells to Th17. Higher antibody titer against P. aeruginosa indicates chronic infection and involvement of the immune system. Our CF patients with isolated P. aeruginosa showed higher percentages of Th17 cells than those with no infection (8.08±3.09% vs. 6.25±2.42%), although the differences were considered non-significant. There are some publications regarding the inability of CF patients to clear P. aeruginosa due to abnormalities in the airway surface liquid and ciliary function which lead to persisting activation of inflammatory pathways that promote the development of Th17 phenotype and chronic IL-17 production.33
The excessive attraction of neutrophils in the airways, along with Th17 cells and cytokines such as IL-17A and IL-17F were shown to be involved in the induction of mucine production, the hyper-contractibility of the airway smooth muscle cells and the corticosteroid resistant airway inflammation in mouse models.10 In our study, we did not find any differences regarding Th17/IL-17A and laboratory, clinical nor instrumental findings of the CF patients. However, our research was devoted to the investigations in the periphery, but future aspects of our work might be more invasive – to assess the Th17 cells and IL-17A in sputum, bronchoalveolar lavage, etc.
ConclusionWe confirmed that the percentage of Th17 cells was significantly increased in the peripheral blood of children with severe BA compared to the children with moderate BA. Most data in the literature, including our results, conclude that in asthma, particularly severe, airway inflammation is not only driven by Th2 but also by Th17 lymphocytes. Having in mind the heterogeneity of the disease, targeting Th17/IL-17 could help treat patients with the most severe symptoms. However, this should be considered with pronounced caution due to their essential physiological functions in the body.
Conflict of interestThe authors have no conflict of interest to declare.
This work was supported by the Medical University of Sofia [grant number 54, 2015].