Few studies on the age of resolution of Food Protein Induced Enterocolitis Syndrome (FPIES) induced by solid foods are available. In particular, for FPIES induced by egg, the mean age of tolerance acquisition reported in the literature ranges from 42 to 63 months.
ObjectiveWe have assessed whether the age of tolerance acquisition in acute egg FPIES varies depending on whether the egg is cooked or raw.
MethodsWe conducted a retrospective and multicentric study of children with diagnosis of acute egg FPIES seen in 10 Italian allergy units between July 2003 and October 2017. The collected data regarded sex, presence of other allergic diseases, age of onset of symptoms, kind and severity of symptoms, cooking technique of the ingested egg, outcome of the allergy test, age of tolerance acquisition.
ResultsSixty-one children with acute egg FPIES were enrolled, 34 (56%) males and 27 (44%) females. Tolerance to cooked egg has been demonstrated by 47/61 (77%) children at a mean age of 30.2 months. For 32 of them, tolerance to raw egg has been demonstrated at a mean age of 43.9 months. No episodes of severe adverse reaction after baked egg ingestion have been recorded.
ConclusionsIt is possible to perform an OFC with baked egg, to verify the possible acquisition of tolerance, at about 30 months of life in children with acute egg FPIES.
Food protein-induced enterocolitis syndrome (FPIES) is a non-IgE-mediated food allergy (FA), which typically occurs during childhood, with episodes of prolonged and repeated vomiting from 1 to 4h after the ingestion of the offending food.1 Vomiting is often accompanied by lethargy and pallor and may be followed by diarrhea.1 The most involved foods are cow's milk and soybean among liquid foods1; rice is the most commonly reported solid food, except in Italy where it is fish.2 In the United States of America (USA), hen's egg is in fourth place, being involved in 11% of cases3; in England it is in third place with 13% of cases4 as well as in Italy with 6% of cases.2 Up to 2014, 62 cases of acute hen's egg-induced FPIES (from now egg FPIES) were published,5 with the largest contribution given by Ruffner et al.3 with 51 cases. USA researchers have reported that, in their case studies, tolerance in children with egg FPIES was reached on average at 42 months. However, Vazquez-Ortiz et al.6 reported that the mean age for achieving tolerance in their eight Spanish cases of egg FPIES was 63 months, as for the eight Australian cases of Lee et al.7 In our previous series, tolerance was demonstrated on average at 53 months.2
In these studies it has not been evaluated whether the mean age of tolerance acquisition in egg FPIES may change depending on whether or not the trigger food is cooked. This evaluation was the primary outcome of our study. Other objectives were the modalities used for the diagnosis by the various allergy units, the relationship between the degree of cooking of the egg or the amount of ingested egg and the severity of episodes of acute FPIES, the number of harmless ingestions before the onset of the egg FPIES, or variability of the severity of the episodes in the single patient.
Materials and methodsWe conducted a retrospective multicentric study in the period between July 2003 and October 2017. All children who had received a diagnosis of egg FPIES in 10 Italian allergy units were enrolled. The diagnosis of FPIES was issued by pediatric allergist based on various patterns of diagnostic criteria published in the literature.1,8–12 The age at diagnosis referred to the time a doctor diagnosed FPIES, while the age of tolerance acquisition referred to the time of the passed oral food challenge (OFC) or to the time of a home ingestion without adverse reactions.
The collected data regard sex, presence of other allergic diseases, age of onset of symptoms, kind and severity of symptoms, cooking technique of the ingested egg, outcome of the allergy test, age of tolerance acquisition.
Allergy testsSkin prick tests (SPT) were performed at the time of diagnosis and before the execution of each OFC, using commercial egg allergen extracts and/or raw and cooked fresh eggs (prick by prick, PbP).
Patients with history of at least two acute episodes consistent with the suspicion of acute FPIES, according to the above criteria,8–12 or with the major criterion plus at least three of the minor criteria according to the International Consensus on FPIES,1 received a definitive diagnosis of FPIES based on clinical history. Patients with only one acute episode and fewer than three minor criteria,1 underwent diagnostic OFC.
Patients who received a definitive diagnosis of FPIES were prescribed an elimination diet for the offending food and were then offered an OFC to verify tolerance acquisition at least 12 months after the occurrence of the last acute episode.
All centers evaluated the OFC as positive (i.e. failed) exclusively basing on clinical signs and symptoms, mainly on the appearance of repeated vomiting and, secondarily, on other signs and symptoms that accompanied vomiting, such as lethargy and pallor. Every pharmacological treatment was administered at the discretion of the attending physician and included intramuscular or intravenous ondansetron and/or saline intravenous bolus and intravenous corticosteroids.
Cooking technique and amount of egg administered during ingestion at home and during the diagnostic and follow-up OFC were recorded.
The severity of the reactions observed during ingestion at home and during the diagnostic and follow-up OFC was differentiated in mild, moderate and severe.1
Statistical analysisData were collected in an Excel database. Results have been expressed as mean value and standard deviation (SD). For each one 95% confidence interval (95% CI), the minimum value and the maximum value has been calculated. The comparison between groups, when it was necessary to demonstrate the difference between the means of different series, was performed by t-Student's test. Proportions were compared by Chi–Quadro test. A value of p<0.05 was considered statistically significant. Statistical analysis was conducted by SPSS software, version 20.0 for Windows.
ResultsSixty-one children with egg FPIES were enrolled, 34 (56%) males and 27 (44%) females. Atopic dermatitis was present in 15 (25%) children, three (5%) children had an IgE-mediated FA to a food other than egg, five (8%) a respiratory allergy. In the first months of life, 42/61 (69%) children took maternal milk, 41/42 (98%) of the feeding mothers ate eggs during the period of lactation. The mean age at diagnosis was 15 months (SD=8.5 months, 95% CI=12.8–17.2 months, minimum–maximum=6–63 months). In 52/61 cases (85%) the diagnosis was made on an anamnestic basis, without resorting to OFC: the diagnostic criteria adopted were in 52% of cases those of Miceli Sopo et al.10; in 15% those of Mount Sinai Hospital11; in 13% those of Leonard et al. of 201512; in 11% those of Nowak-Węgrzyn et al.1; in 8% those of Leonard et al. of 2012.9 In 9/61 (15%) the diagnosis was made by OFC. Episodes of acute FPIES before the diagnosis were on average 2.3 (SD=0.9; 95% CI=2.1–2.5, minimum–maximum=1–4). In 53/61 (87%) children FPIES was caused only by egg (single FPIES), in 8/61 (13%) children it was also caused by other foods (multiple FPIES: two patients also had FPIES from chicken meat, three from cow's milk, two from fish, one from mollusk). No patient had a chronic symptomatology caused by egg.
All patients performed SPT and/or PbP with egg: skin tests were positive in 9/61 (15%) children (atypical FPIES).
Pre-diagnosis episodes of acute FPIESBefore the diagnosis was formulated, at least two episodes of acute FPIES were presented by 51/61 (83%) children, 20/61 (33%) children presented at least three episodes. The characteristics of the episodes were similar to those already known of acute FPIES from any other food (Table E1 available online). In particular, the mean age at the 1st episode was 9.8 months (SD=3.8 months, 95% CI=8.8–10.7, min–max=6–26 months). The cooking techniques used were: raw egg mixed in boiling broth (the most frequently used), raw egg, omelet fried in the pan for 3min, soft-boiled egg (i.e. boiled for 5min), hard-boiled egg (i.e. boiled for 10min), baked omelet, desserts containing baked egg (biscuit, muffin, an Italian dessert called ciambellone). The amount of egg taken during the pre-diagnosis episodes was variable: a whole egg, half an egg, two teaspoonfuls, a teaspoonful, only the yolk.
The variability of the severity of the episodes in the single patient was evaluated in the 20 patients with at least three episodes: the physician's opinion about the severity of each single episode changed in 50% of the cases, in the remaining 50% it remained the same from the 1st to the 3rd episode. The severe grade was assigned at least once to 15 patients. We have not observed a relationship with the amount of ingested egg (from a teaspoonful to a whole egg) or with the degree of cooking, except for the fact that ingestion of baked egg never caused an acute episode classifiable as severe.
The first ingestion of egg was the cause of acute FPIES in 39/61 (64%) children. Instead, 22/61 (36%) children had ingested egg without presenting any adverse reaction before the onset of FPIES, on average 4.3 times (SD=1.6; 95% CI=3.7–5 times, minimum–maximum=1–10). Degree of cooking and amount of egg during harmless pre-FPIES ingestions were variable. In some cases, they were equal or lower than in the ingestion that caused acute FPIES.
Diagnostic OFCA diagnostic OFC was performed in 9/61 (15%) children at the mean age of 19.4 months (SD=8.8; 85% CI=13.3–25.5 months, minimum–maximum=13–40). The mean distance from the last episode of egg FPIES was 8.5 months (SD=9.5; 95% CI=2.3–14.7 months, minimum–maximum=1–29). Egg was administered raw in five cases, baked in wheat matrix in two, boiled for 10min (hard boiled) in one case, and in the form of omelet fried in the pan for three minutes in one case. Four children were given half an egg, three a whole egg, two a teaspoonful of egg. With regard to the methods of administration, in four cases the indications of the International Consensus on FPIES were followed (three equal doses over 30min)1; in three cases egg was administered in a single dose; in one case, due to the positive prick test, OFC was conducted with incremental doses as for IgE-mediated FA13; in one case, a dose equal to one tenth of an egg was first administered, the remaining nine tenths after one hour. Vomiting occurred on average after 2.1h (SD=1.3; 95% CI=1.3–3.9h, minimum–maximum=1–5). The mean number of vomitings was 3.1 (SD=1.5; 95% CI=2.1–4.1, minimum–maximum=1–5). The grading of the severity of the reaction was moderate in five cases, severe in two (which also presented systolic hypotension and hypothermia), and mild in another two. No clear associations were observed between degree of cooking, amount of egg and severity of the observed reaction. The two cases considered severe occurred as a result of the intake of a whole raw egg; of the two cases judged to be mild, one occurred with a teaspoonful of raw egg and one with a whole egg in the form of omelet fried in the pan for three minutes; of the five judged moderate, two took place with a whole raw egg, two with half an egg baked in wheat matrix and one with a teaspoonful of boiled egg. Furthermore, no relationship between the severity of the previous episode and the severity of the reaction during the OFC was observed. In fact, the severe grade for the adverse reaction in OFC was attributed to two children who had presented a moderate reaction in the first episode, while one case that had been judged to be severe at the first episode after taking a raw whole egg mixed with boiling broth, presented a moderate reaction during OFC with a whole raw egg. The pharmacological therapy always included fluids and intravenous steroids (except in the cases judged to be mild, who received no treatment), one case also received intravenous ondansetron, and one case intramuscular adrenaline.
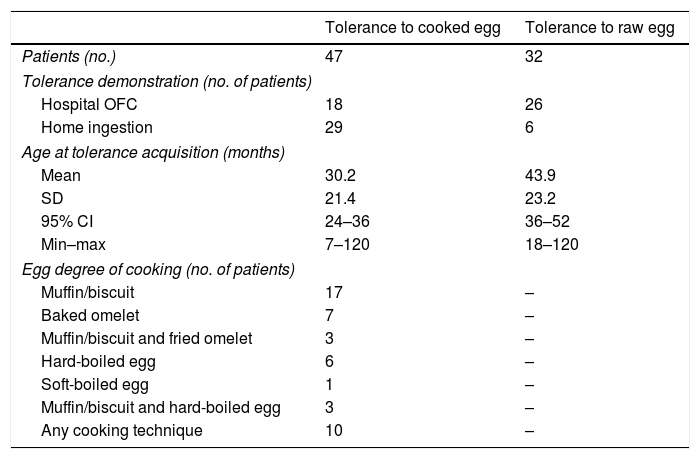
Tolerance acquisitionTable E2 (available online) shows the data concerning the OFC performed to evaluate the eventual tolerance acquisition. We lack data about possible tolerance acquisition for 14/61 (23%) children: they did not perform follow-up OFC and their parents did not refer any accidental ingestion after their last known episode of egg FPIES.
Tolerance to cooked egg (regardless of cooking technique) was achieved by 47/61 children (77%) at the mean age of 30.2 months (see Table 1). All these 47 children ate at least half an egg, and the majority of them ate an entire egg, at the age to which the acquisition of tolerance was attributed.
Tolerance acquisition in 47 children with egg FPIES.
| Tolerance to cooked egg | Tolerance to raw egg | |
|---|---|---|
| Patients (no.) | 47 | 32 |
| Tolerance demonstration (no. of patients) | ||
| Hospital OFC | 18 | 26 |
| Home ingestion | 29 | 6 |
| Age at tolerance acquisition (months) | ||
| Mean | 30.2 | 43.9 |
| SD | 21.4 | 23.2 |
| 95% CI | 24–36 | 36–52 |
| Min–max | 7–120 | 18–120 |
| Egg degree of cooking (no. of patients) | ||
| Muffin/biscuit | 17 | – |
| Baked omelet | 7 | – |
| Muffin/biscuit and fried omelet | 3 | – |
| Hard-boiled egg | 6 | – |
| Soft-boiled egg | 1 | – |
| Muffin/biscuit and hard-boiled egg | 3 | – |
| Any cooking technique | 10 | – |
Abbreviations: OFC, oral food challenge; SD, standard deviation.
For 12/47 (25.6%) who tolerated baked egg (mainly in wheat matrix) we have no information about the possible acquisition of tolerance to raw egg or less extensive cooking methods than the baked one. 32/47 (68%) children who had acquired tolerance to cooked egg showed to have also achieved tolerance to raw egg at the mean age of 43.9 months. For these 32 children, the difference between the mean age in which the tolerance to cooked egg was demonstrated and the mean age in which the tolerance to raw egg was demonstrated is equal to 12.7 months (p=0.004). 3/47 (6.4%) children were still documented as allergic to raw egg after acquiring tolerance to cooked egg. Two of them were atypical egg FPIES. Furthermore, for 10 of the 47 children who tolerated cooked egg we are not sure that they have ever been allergic to it. In fact, they had presented episodes of acute FPIES with raw or very little cooked egg (such as raw egg mixed with boiling broth) and had never ingested either a moderately or well-cooked egg.
Atypical FPIES (nine children, 15%) was not associated with statistically significant differences with respect to the mean age of achievement of tolerance or to the percentage of tolerant children. In fact, children with atypical FPIES achieved tolerance to cooked egg on average at 33.4 months (SD=23.5, 95% CI=13–60 months, minimum–maximum=7–62), while children with classical FPIES achieved tolerance to cooked egg at a mean age of 40 months (SD=23, 95% CI=23–36 months, minimum–maximum=8–120) (p=0.73). Children with atypical FPIES achieved tolerance to raw egg at 47.5 months (SD=10.5, 95% CI=37–57 months, minimum–maximum=32–62), while children with classical FPIES achieved tolerance to raw egg at 43.4 months (SD=24.6, 95% CI=34–52 months, minimum–maximum=18–120) (p=0.57). Children with atypical FPIES who resulted tolerant to cooked egg were 44%, those with classical FPIES were 69% (p=0.137). Children with atypical FPIES tolerant to raw egg were 44% while those with classical FPIES were 52% (p=0.64). Only one patient, with atypical FPIES, turned his reactivity toward an IgE-mediated form, manifesting anaphylaxis during an OFC with raw egg at the age of 69 months.
Sex, presence of any atopic condition and number of foods involved (single FPIES vs. multiple FPIES) were not associated with the probability of acquiring tolerance.
DiscussionOur study had as its primary objective to provide information about the mean age of tolerance acquisition in children with egg FPIES, in particular in relation to degree of cooking of the culprit food. To date we know that in USA3 the mean age of tolerance acquisition was 42 months, in Spain6 and Australia7 the age is shifted to 63 months, in Italy we observed a mean age of 53 months2: the latter three studies2,6,7 had studied few children, and this makes their estimates inaccurate.
In addition, regarding the age of tolerance acquisition, to date no difference has been studied between cooked egg and raw egg. It is widely demonstrated that processing egg by cooking can change its allergenicity in IgE-mediated egg allergy.14 In FPIES this aspect is less explored and the current dietary indication1,15 includes that children affected by egg FPIES must be educated to avoid both raw and cooked egg, and that any attempts to administer cooked egg should be done in the hospital. In fact, the reports in the literature about the tolerability of cooked egg in children with egg FPIES are not encouraging: for example, Mehr et al.16 reported that four of the five children with egg FPIES that had exposure to cooked egg reacted to it, while the 12 children affected by FPIES by cow's milk who had exposure to cooked milk tolerated it.
In our study, the mean age of acquisition of tolerance to cooked egg, which involved 47/61 (77%) of our patients, was 30.2 months. Tolerance to raw egg has been demonstrated at the mean age of 43.9 months for 32 of them. In truth, however, we can affirm that tolerance to cooked egg has been achieved before the one to raw egg for only 3/47 children. In fact, we cannot know if the remaining 44/47 children were still allergic to raw egg when they showed tolerance to cooked egg. It cannot be excluded that the fact that for 32 of them the mean age of acquisition of tolerance to raw egg was significantly higher is more likely to be due to the fear of parents (and perhaps doctors) to also administer raw egg immediately, considered by them, perhaps rightly, more allergenic than cooked egg, even in the case of FPIES. Despite this important doubt, it is certain that, in our population, 77% of children with egg FPIES, therefore a considerable percentage, tolerate at least cooked egg at a mean age of a little more than two and a half years.
Basing on our data, we are not able to show if one cooking technique rather than another makes the egg more or less tolerable. Although, at least theoretically, the higher the grade and the longer the time of cooking is, the greater the probability should be that the child tolerates egg, as for IgE-mediated egg allergy.14
The practical consequence of our study is that an OFC for the verification of the possible acquisition of tolerance to cooked egg could be offered before three years of age. We suggest starting with the OFC with baked egg, since in our population its ingestion has not been associated with reactions considered severe by the physician. For those children who presented episodes of acute FPIES exclusively by raw or very little cooked egg, the OFC with baked egg could be done even immediately, without waiting for 30 months of age. In fact, it is not said that anyone who is allergic to raw egg must necessarily be allergic also to cooked egg.
In our study the severity of reactions during OFC did not show a relationship with the amount of egg ingested, nor with the degree of cooking of egg, nor with the severity of previous reactions. This may affect the recommendation to take special precautions for children with a history of severe reactions or reactions to very small doses. We do not consider necessary in these children to administer a dose up to 10 times lower or divide the calculated food dose into three equal doses to be administered over 30min: we propose to administer the whole calculated dose and then wait for at least four hours, without differences depending on clinical history. It is relevant that the ingestion of baked egg has never caused episodes definable as severe: therefore, it could be reasonable to perform the OFC with baked egg first, as already written.
In conclusion, the retrospective and multicentric study of our case series of 61 Italian children with egg FPIES, allowed us to observe that the majority of patients achieve tolerance, at least to cooked egg, at an age of about 30 months. Furthermore, we could observe that the ingestion of baked egg has never resulted in severe adverse reactions. Then, we suggest performing an OFC with baked egg at about two and a half years of life.
Author contributionsStefano Miceli Sopo and Giulia Bersani conceived the design of the study and drafted the article.
Laura Badina, Giorgio Longo, Giovanna Monti, Serena Viola, Salvatore Tripodi, Giuseppe Barilaro, Iride Dello Iacono, Carlo Caffarelli, Carla Mastrorilli, Simona Barni, Francesca Mori, Lucia Liotti, Barbara Cuomo, Fabrizio Franceschini, Domenico Viggiano acquired the data.
Alberto Romano and Serena Monaco analyzed the data and interpreted them.
Claudia Fantacci revised the article.
All authors gave final approval of the version to be published.
Funding sourceNone.
Declaration of interestNone.
We declare that the work described has not been published previously, that it is not under consideration for publication elsewhere, that its publication is approved by all authors and tacitly by the responsible authorities where the work was carried out, and that, if accepted, it will not be published elsewhere in the same form, in English or in any other language, including electronically without the written consent of the copyright-holder.