Del-Rio Camacho and colleagues reported that component-resolved diagnosis (CRD) can influence the choice of allergen immunotherapy (AIT) as they evaluated a group of 70 allergic children with poly-sensitization.1 In particular, a small panel of allergen molecules may be sufficient in clinical practice.
It is well known that CRD may remarkably influence AIT prescription, mainly in poly-sensitized patients.2 Grass allergy is very common in Europe, and a recent study investigated the molecular pattern of 11,235 Italian patients with allergic rhinitis.3 That study showed that sensitization to Phl p 1 was the most common, but that there was a significant variation of distribution across Italy. Furthermore, sensitizations to pan-allergens Phl p 7 and 12 were also significantly ranged in the different geographic areas.
On the basis of this background, the aim of the current observational experience was to define the molecular profile of a group of Italian children allergic to grass pollen and treated with AIT and to characterize the impact on response to AIT.
Globally, 76 children (46 males and 30 females; mean age 12.4±2.4 years) were enrolled in the study. They were visited at five Italian Pediatric Allergy centers. The Review Boards of each center approved the procedure. The patients’ parents gave written informed consent.
Inclusion criteria were: (i) pediatric age; (ii) both genders; (iii) written informed consent signed by the patients’ parents; and (iv) satisfaction of criteria for reimbursement by Italian Health Service (including: history of moderate-severe allergic rhinitis for at least two years, positive skin prick test to grass pollen, serum specific IgE to grass pollen ≥2 Cap System, mono-allergy to grass pollen, and no past AIT). Exclusion criteria were: (i) allergy to other allergens; (ii) uncontrolled asthma; (iii) immunodeficiency; (iv) cancer; and (v) oral disorders. The therapeutic program for AIT tablets for grass pollen allergy fulfilled the rules established by the Italian Agency for Drugs. In particular, children were treated with the grass allergen tablet Grazax® (Phleum pratense 75,000 SQ-T/2800 BAU, ALK, Denmark). All children initiated the treatment at least two months before the beginning of the 2017 grass pollen season. Children were visited before treatment and after one year of treatment.
AIT response was defined according to validated criteria, such as a clinically relevant (such as at least <30%) reduction of the combined score for symptoms and medication use after AIT.4
Before AIT, the sensitization molecular profile was detected by the ISAC method measured by ImmunoCAP solid-phase allergen chip (ISAC) test according to the manufacturer's recommendations (Thermo-Fisher Italy, Milan, Italy) and as previously reported (7). The ISAC score was reported as ISAC Standardized Units (ISU), which ranges from 0 to 100. Data were obtained by specific software. Sensitization was defined if the value was >0.3ISU/mL. IgE to Phl p 1, 2, 4, 5, 6, 7, 11, and 12, and Cyn d 1were assessed.
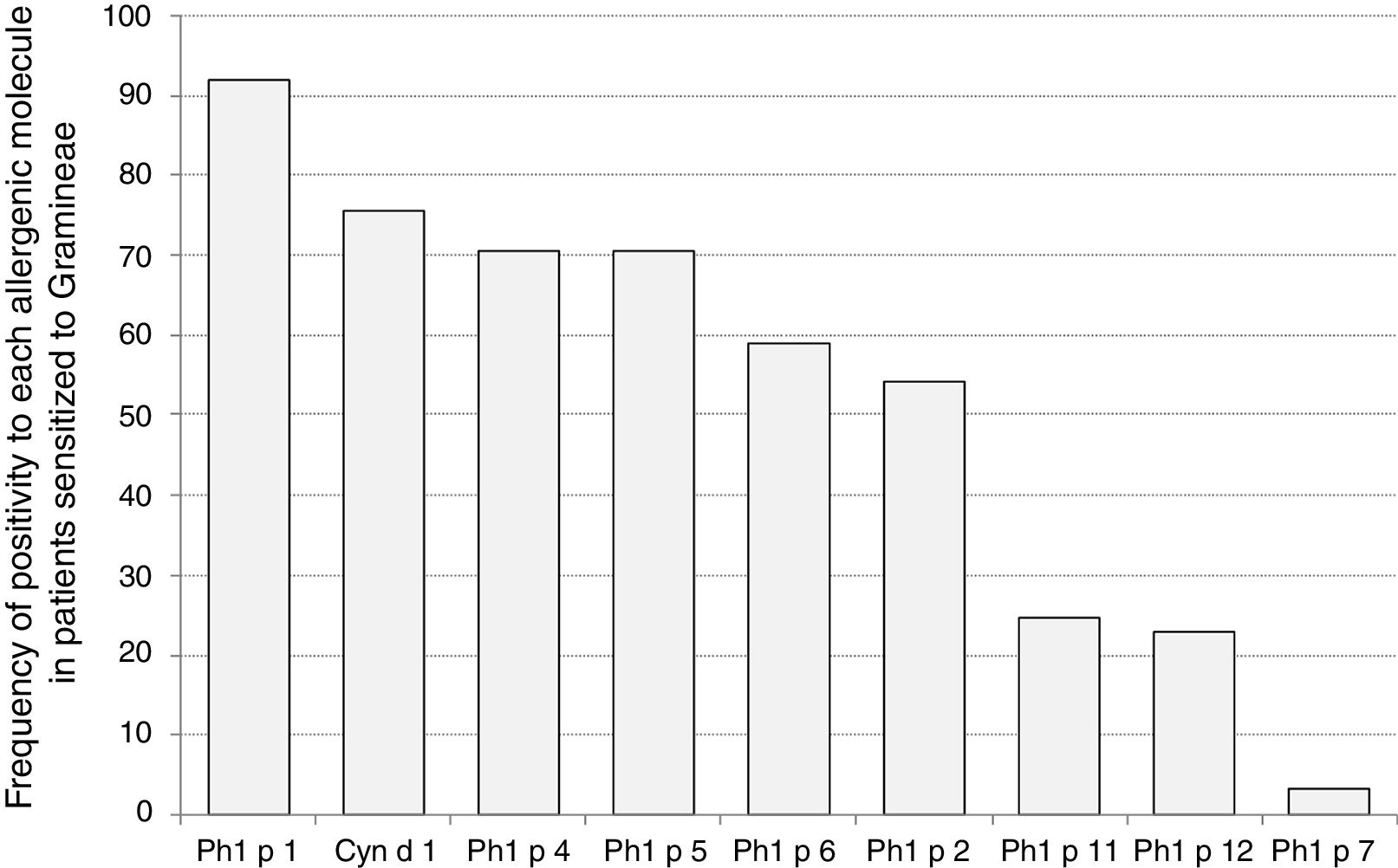
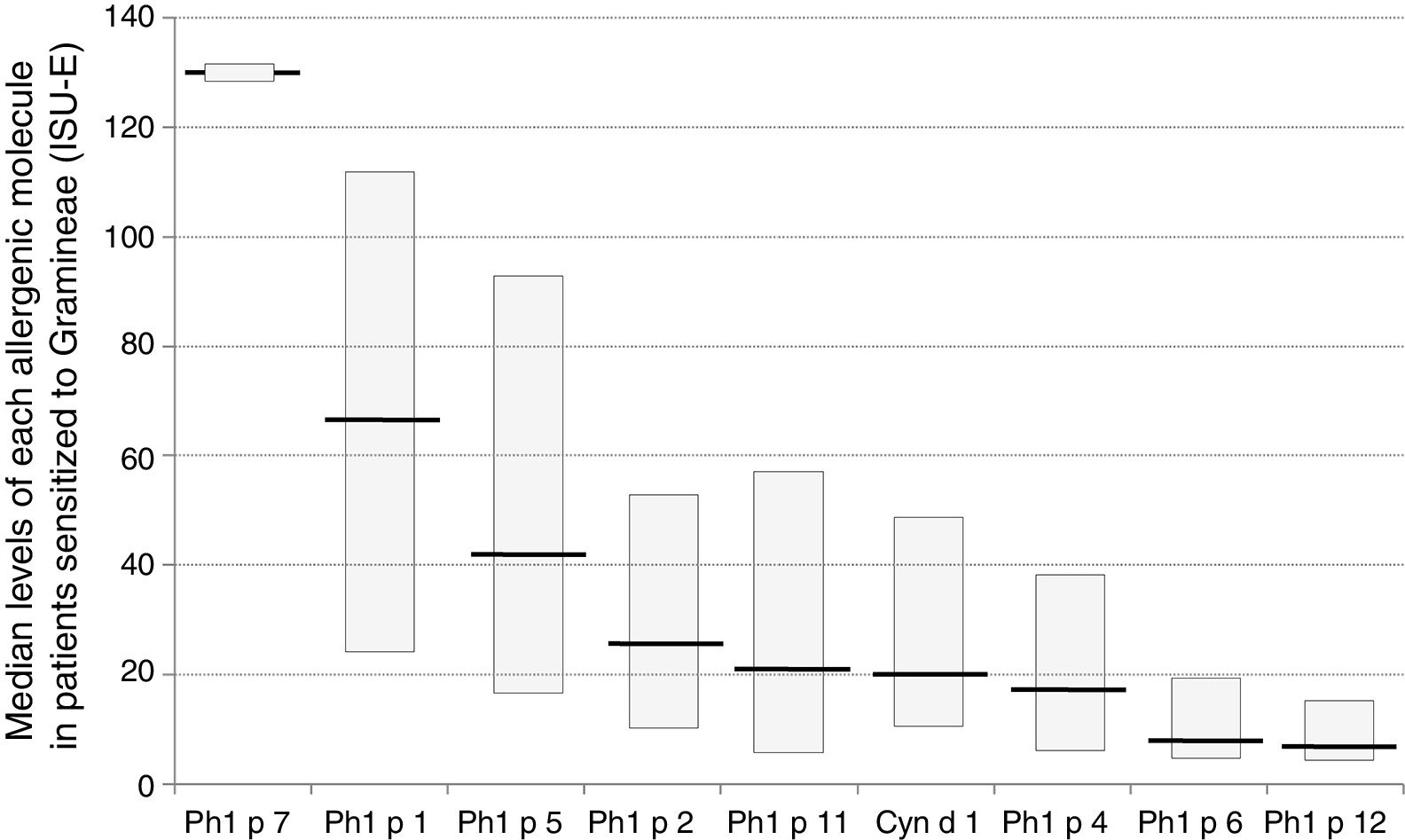
Sensitization to Phl p 1 was the most common (92%), followed by Cyn d 1 (75%), Phl p 4 (71%), and Phl p 5 (70%), as reported in Fig. 1. The pan-allergens Phl p 12 and 7 caused sensitization in 17 and two children respectively, but never as mono-sensitization. In other words, all children were sensitized to at least one genuine molecule of the grass pollen allergens. Interestingly, the highest IgE levels concerned Phl p 7 and Phl p 1, whereas the lowest ones concerned Phl p 12, as reported in Fig. 2.
After one-year AIT treatment, all children responded to AIT on the basis of a reduction of combined score <30% of baseline values. Notably, the sensitizations to the pan-allergens did not affect the AIT response, most likely because all patients were sensitized to the genuine grass molecules.
Therefore, the current experience confirms the relevance of molecular diagnostics in the work-up and management of allergic patients, namely for AIT prescription. In particular, the presence of co-sensitization to pan-allergens, i.e. polcalcin and profilin, should not dissuade from prescribing AIT. Of course, molecular findings should be adequately interpreted by a well-trained specialist.
In conclusion, AIT for grass pollen may be effective in patients with sensitization to genuine molecular allergens also in the presence of pan-allergen co-sensitization. Therefore, co-sensitization to pan-allergens is not a contraindication for AIT in grass-allergic patients.