Atorvastatin is a statin group medicine that reduces the level of serum cholesterol; thus it is used to treat hypercholesterolaemia. Independent of the cholesterol-lowering property of statins they also have anti-inflammatory and immunomodulating effects. This study aimed to investigate the effect of atorvastatin on histological changes in the lungs in a murine model of chronic asthma.
Materials and methodsTwenty-eight BALB/c mice in Group I, II, III and IV were divided into four groups. All the mice except the control group (Group I) were sensitised with ovalbumin. Intraperitoneal injection with saline, atorvastatin (10mg/kg), dexametazon (1mg/kg) was administered to Group II, Group III, and Group IV respectively for five consecutive days. Mice were sacrificed 24h after the last drug administration. All the histological properties of lung tissue samples from all groups were evaluated with light and electron microscopy. In addition, IL-4 and IL-5 levels of the lung tissue were measured.
ResultsWhen Group II and Group III (atorvastatin) were compared, thicknesses of basement membrane and subepithelial smooth muscle layer, height of epithelium, number of mast and goblet cells were significantly lower in Group III. In comparing Group III (atorvastatin) and Group IV (dexamethasone), all the improvements in histological parameters were similar. In addition, the IL-4 and IL-5 levels of the lung tissue were significantly lower in atorvastatin group (Group III) compared to placebo-treated group.
ConclusionAtorvastatin had a beneficial effect on histological changes in a chronic murine model of asthma.
Asthma is formed by airway hyperresponsiveness to airflow obstruction and airway remodelling. This chronic inflammatory is most common in childhood. In the last decade the morbidity and mortality of asthma has been increasing.1
The current approach for asthma treatment aims to suppress airway inflammation. The most common drugs in asthma therapy are inhaled glucocorticoids in spite of several side effects when they are used in high doses or for a prolonged time.2 Airway remodelling consists of progressive structural changes in the composition, content, and organisation of the cellular and molecular constituents of the airway wall.3 Although current asthma therapies are effective in reducing inflammation, airway remodelling is poorly responsive to current therapies.4,5 New therapy strategies which have a potent effect on airway remodelling are required.
Statins reduce the biosynthesis of cholesterol by inhibiting 3-hydroxy-3-methylglutaryl-coenzyme A reductase. They were mostly used in patients with high cholesterol level to reduce the morbidity and mortality of coronary artery disease.6 Statins have been recently recognised as anti-inflammatory agents and shown to possess immunomodulating effects.7,8 Statins also have a potential therapeutic role in respiratory disease. Bronchial smooth muscle hyperresponsiveness induced by ovalbumin stimulation has been found to be significantly attenuated by an intraperitoneal injection or inhalation of statins.9,10
However, the accurate benefits of atorvastatin in asthma treatment have not been investigated yet. Therefore, in this study our aim is to investigate the efficacy of atorvastatin on lung histopathology in a murine model of chronic asthma.
Materials and methodsExperimental animalsTwenty-eight conventionally raised, 6- to 8-week-old male BALB/c mice weighing 18–20g were used for the study. They were maintained in the experimental animal laboratory of Dokuz Eylul University and kept in hygienic macrolene cages and fed a standard laboratory diet ad libitum in air-conditioned rooms on a 12h light/12h dark cycle. All experiments were carried out according to the protocols approved by the local animal use and care committee (Dokuz Eylul University). The protocols complied with the standards in the Guide for the Care and Use of Laboratory Animals, prepared by the Institute of Laboratory Animal Resources and published by the National Academy Press, National Research Council, Commission of Life Sciences, Institute of Laboratory Animal Resources.
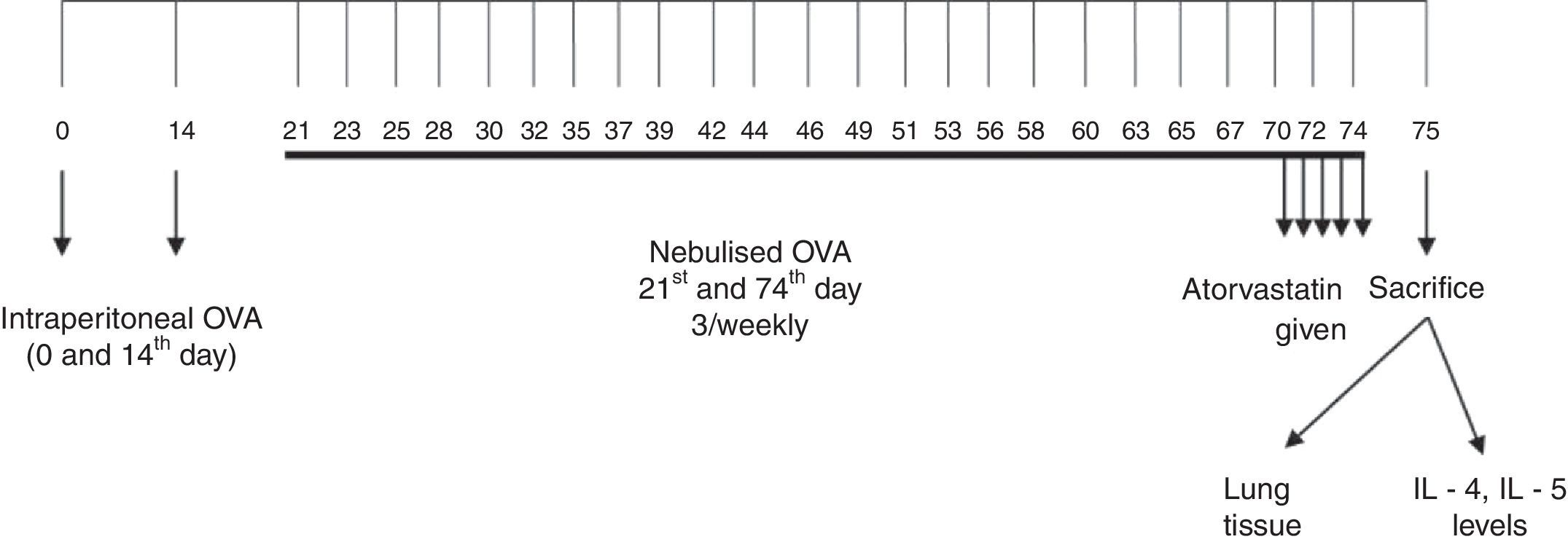
Sensitisation and inhalational exposureMice were divided into four groups. Mice in the study groups except for the control group were sensitised on days 0 and 14 by intraperitoneal (i.p.) injections of 10mg chicken egg albumin (ovalbumin, grade V, 98% pure; Sigma, St. Louis, MO, USA) with alum as an adjuvant. Mice in study Groups II, III, and IV were then challenged with an aerosol of 2.5% ovalbumin in saline for 30min/day for three days of the week for eight weeks beginning from the 21st day of the study. Mice in control group (Group I) received normal saline with alum i.p. on days 0 and 14 of the experiment and aerosolised saline without alum for 30min/day for three days of the week for eight weeks beginning from the 21st day of the study11 (Fig. 1). Exposures were carried out in a whole body inhalation exposure system. Temperature and relative humidity were maintained at 20–25°C and 40–60%, respectively. A solution of 2.5% ovalbumin in normal saline was aerosolised by delivery of compressed air to a sidestream jet nebuliser and injected into a chamber. The aerosol generated by this nebuliser comprised >80% particles with a diameter of <4mm. Particle concentration was maintained in the range of 10–20mg/mm3.12
Study drugsMice in Group I received saline; Group II received atorvastatin at dose of 10mg/kg/day, and Group III dexamethasone at dose of 1mg/kg/day intraperitoneally once a day for the last five days of the challenge period. Intraperitoneal doses of atorvastatin (Ator, Sanovel, Turkey) and dexamethasone 1mg/kg were chosen from other studies also conducted with BALB/c mice.13
Histopathological analysisAnimals were sacrificed by an overdose of ketamine 24h after the last drug administration. Two investigators who were blinded to the treatment groups interpreted the histopathology. Tissue specimens were obtained from the mid zone of the left lung of mice. Samples were fixed in 10% formalin for light microscopic evaluation. Some tissue samples of 1–2mm3 obtained from adjacent regions were stocked in 2.5% gluteraldehyde for electron microscopic evaluation. After fixation, samples were embedded in paraffin for light microscopic evaluation and serial sections of 5-μm thickness were prepared. After choosing the first section randomly, 10 sections in each mouse were selected by skipping over 10 sections and proceeded to the staining process. For light microscopic evaluation, three different staining processes were used. The first 10 samples were stained with haematoxylin and eosin (H&E). In these samples, general tissue features were examined and thicknesses of epithelium and subepithelial smooth muscle layers of the medium and small airways were measured. In order to evaluate the thicknesses of epithelium and subepithelial smooth muscle layers, measurements were performed from four points of each airway at levels of 3, 6, 9, and 12 o’clock. Considering that each section contained approximately two to three airways, around 20 or more airways were evaluated for each mouse.
Photomicrographs were taken by JVC TK-890-E camera (Japan), which was adapted on an Olympus BH-2 RFCA model microscope (Olympus Optical, Tokyo, Japan). The histological analysis was carried out with UTHSCSA Image Tool for Windows Version 3.00 software.
The consecutive 10 sections were stained with toluidine blue and the other 10 sections with periodic acid-Schiff (PAS). Photomicrographs were taken randomly from five fields of each section which were stained with toluidine blue. For mast cell enumeration, a standard transparent counting frame representing an area of 20,000μm2 was used manually and eight fields in each photograph were examined for each mouse. Goblet cells stained with PAS were enumerated in 10 sections of each mouse. In each section, three to five randomly selected airways were photographed. Circumferences of all airways were measured and goblet cell numbers in these areas were recorded. For standardisation, goblet cell numbers in 100μm were analysed by division of total goblet cell number to the total length of airway circumferences and multiplying the result by one hundred.
Tissues were embedded in EPON after follow-up process of electron microscopic evaluation. Airways were marked from the semithin sections by light microscope. Ultrathin sections were obtained and stained with uraniyl acetate and lead citrate. Libra 120 Carl Zeiss EFTEM electron microscope (Oberkochen, Germany) was used for this evaluation.
For each mouse, five to seven ultrathin sections were achieved from each two blocks for evaluation of epithelium of the airway, the surrounding structures, and the intercellular connections. For each mouse, eight to ten areas were photographed by Trondle (2048×2048pixel) digital camera, attached to the electron microscope. Thicknesses of the basement membrane of the respiratory epithelium were measured from 20 points of preparations at equal distances to each other and the data were recorded in sequence.
Preparations of lung homogenatesAnimals were sacrificed by an overdose of ketamine 24h after the last drug administration. Lungs were removed and washed with cold PBS three times in order to remove blood. Lung tissues were taken into 2ml microcentrifuge tubes and stored at −80°C until analysis. On the study day, frozen lungs were thawed, weighed (60–80mg), transferred into different tubes on ice containing 5ml of stainless beads, 0.1%SDS, protease inhibitor cocktail (Sigma–Aldrich, St. Louis, MO, USA) and 0.1mg/ml phenylmethanesulfonyl fluoride (PMSF) in PBS. Microcentrifuge tubes were transferred to pre-cooled Tissuelyser LT racks and placed into TissueLyser (Qiagen, Germany) homogenisator. Frequency and time were set to 50 and 5min, respectively. The homogenates were then centrifuged at 15,000×g for 1h at 4°C. The supernatants were stored at −80°C.
Measurement of cytokinesLevels of IL-4 and IL-5 were quantified in the supernatants of the lung tissue by standard ELISA protocols using commercial mouse IL-4 and IL-5 (Bender MedSystems, MedSystems Diagnostics GmbH, Vienna, Austria).
Statistical analysisSPSS 11 package program was used for the statistical analysis. Data were presented as mean±standard deviation (SD) (minimum-maximum) of seven animals in each group. The comparisons between all groups were conducted using the Kruskal–Wallis method. When differences were statistically significant, Mann–Whitney U test was used for group comparisons. p<0.05 was considered statistically significant.
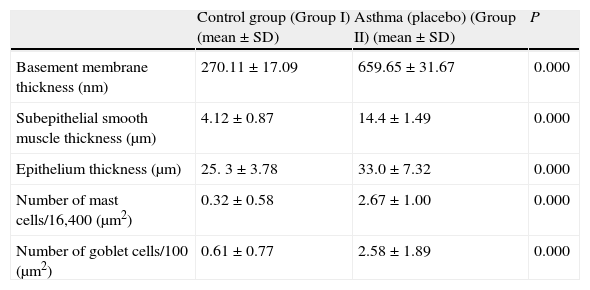
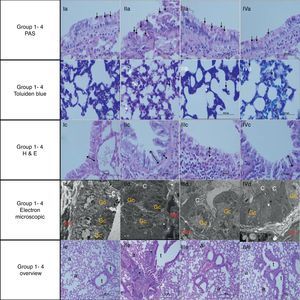
ResultsThe light and electron microscopic examinations revealed normal findings in the control group (Fig. 2). In the chronic asthma group (placebo), the numbers of mast cells and goblet cells as well as the thicknesses of basement membrane, epithelium, and subepithelial smooth muscle layer were significantly higher when compared to the control group (Table 1). Additionally, Fig. 2 demonstrates that characteristic asthmatic changes were successfully established in the group treated with saline (Table 1).
Comparison between asthma (Group I) and control group.
| Control group (Group I) (mean±SD) | Asthma (placebo) (Group II) (mean±SD) | P | |
| Basement membrane thickness (nm) | 270.11±17.09 | 659.65±31.67 | 0.000 |
| Subepithelial smooth muscle thickness (μm) | 4.12±0.87 | 14.4±1.49 | 0.000 |
| Epithelium thickness (μm) | 25. 3±3.78 | 33.0±7.32 | 0.000 |
| Number of mast cells/16,400 (μm2) | 0.32±0.58 | 2.67±1.00 | 0.000 |
| Number of goblet cells/100 (μm2) | 0.61±0.77 | 2.58±1.89 | 0.000 |
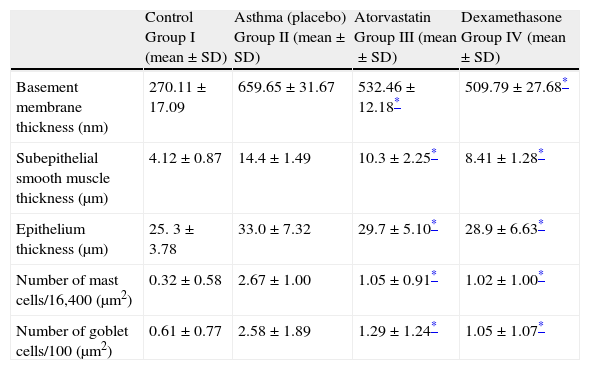
In Group III, the numbers of mast cells and goblet cells as well as the thicknesses of basement membrane, epithelium, and subepithelial smooth muscle layer were significantly lower as compared to the placebo group (Group II) (Table 2 and Fig. 2). This improvement was similar to dexamethasone, the gold standard treatment of asthma (Fig. 2).
Comparison between study groups.
| Control Group I (mean±SD) | Asthma (placebo) Group II (mean±SD) | Atorvastatin Group III (mean±SD) | Dexamethasone Group IV (mean±SD) | |
| Basement membrane thickness (nm) | 270.11±17.09 | 659.65±31.67 | 532.46±12.18* | 509.79±27.68* |
| Subepithelial smooth muscle thickness (μm) | 4.12±0.87 | 14.4±1.49 | 10.3±2.25* | 8.41±1.28* |
| Epithelium thickness (μm) | 25. 3±3.78 | 33.0±7.32 | 29.7±5.10* | 28.9±6.63* |
| Number of mast cells/16,400 (μm2) | 0.32±0.58 | 2.67±1.00 | 1.05±0.91* | 1.02±1.00* |
| Number of goblet cells/100 (μm2) | 0.61±0.77 | 2.58±1.89 | 1.29±1.24* | 1.05±1.07* |
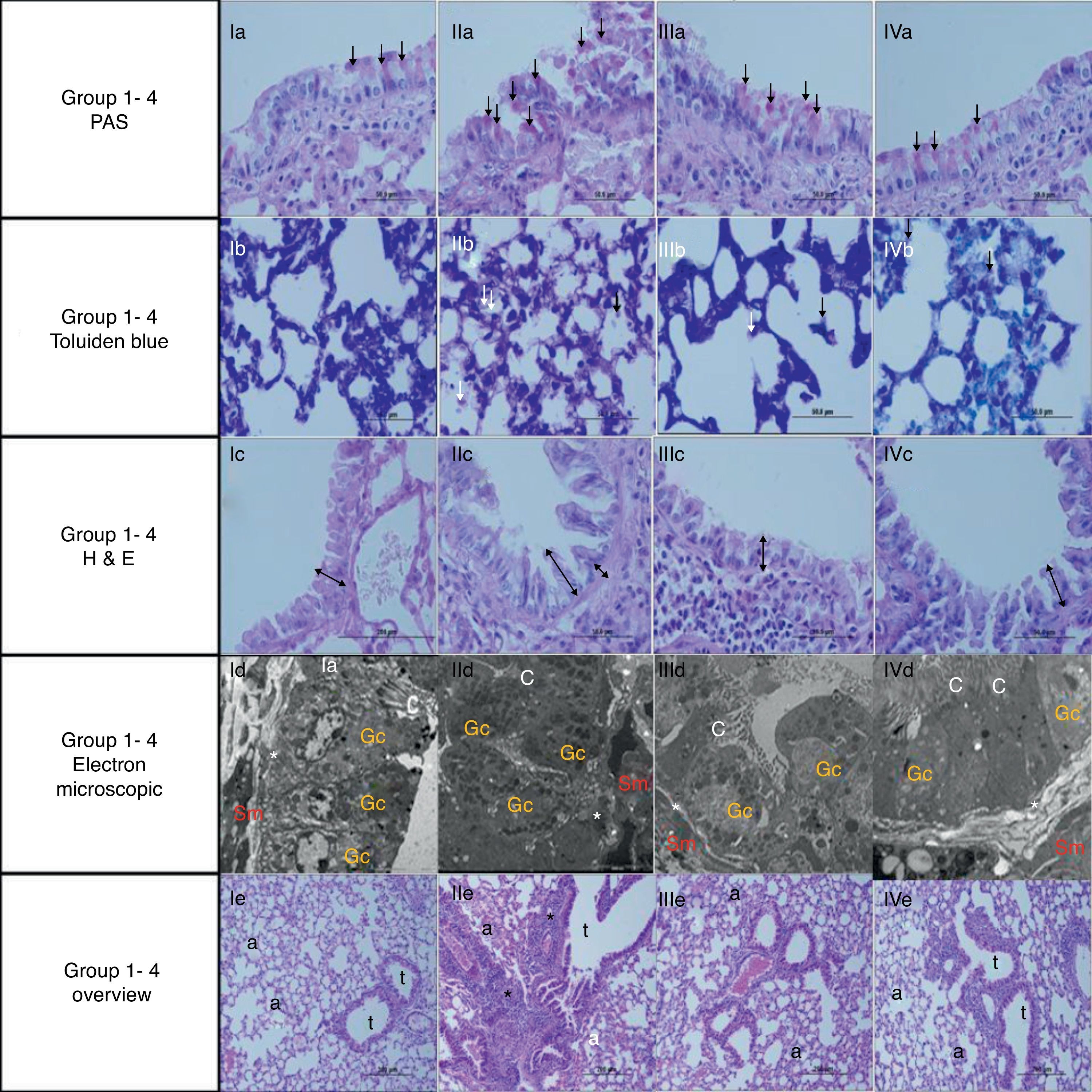
Light and electron microscopic findings of Groups. I, control; II, asthma; III, atorvastatine; IV, dexa group. In representative histological images, lung tissues were stained with PAS (a, 1st row), toluidine blue (b, 2nd row) and H&E (c, 3rd row). In control group, light microscopic findings (Ic) revealed a regular respiratory epithelium and airways (Av), a thin, regular subepithelial smooth muscle layer (arrow with two heads), and normal PAS-stained parenchymal structures (Ia). Parenchymal structures with toluidine blue staining were regular (Ib). Electron microscopy (Id) revealed goblet cells (Gc), and healthy epithelial cells with cilia (C). The basement membrane was thin and regular (*), and there were two to three layers of subepithelial smooth muscle cells (Sm). In the asthma group, H&E staining (IIc) revealed thickened epithelium, thickened subepithelial smooth muscle (arrow with two heads), and peribronchial mononuclear infiltration (*). High numbers of goblet cells (Gc) were seen with PAS-staining (IIa), and mast cells with toluidine blue staining (IIb). In the dexa group, no difference in light microscopic findings was found compared with control rats (IVa, IVb, IVc and IVd). In the atorvastatin group, the pathological changes, cellular infiltration around airways, goblet cell hyperplasia and number of mast cells, were significantly less than in the asthma group. Electron microscopic findings revealed a thickened basement membrane (*) and smooth muscle cells (Sm) in the asthma group (IIId). Healthy respiratory epithelium with cilia (C) and goblet cells (Gc) in the atorvastatine (IIId) and dexa (IVd) groups.
In Group IV (dexamethasone 1mg/kg), all histological parameters were significantly better compared to the group treated with saline (Group II) (Table 2 and Fig. 2).
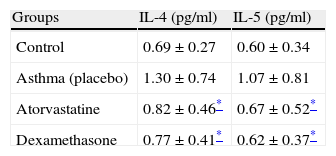
Interleukin 4 and 5 levels were significantly higher in the placebo group compared to the control group. However, significant decreases in the levels of IL-4 and IL-5 were found in the atorvastatin group and the dexamethasone group as compared to the placebo group (Table 3).
DiscussionAsthma is a chronic inflammatory disease characterised by inflammation of the airways, remodelling and decreasing in lung function.14 Suppressing the inflammation in the airways, the inhibition remodelling process and decreasing the symptoms are current therapeutic modalities. Although current therapies reduce the inflammation, their effects to the airway remodelling are not enough. That is why alternative treatment modalities suppressing the inflammation, preventing remodelling and having no side effects (mostly non-steroidal) are needed.
Asthma models mimicking the remodelling process in humans are successfully well established in animal studies.11 The asthma model used in our study is one of the most common models. In our study, histological findings supporting the asthma are basal membrane thickness, goblet cell hyperplasia, smooth muscle thickness and the number of mast cells and epithelial levels were significantly higher in a mouse model of asthma by giving ovalbumin rather than placebo-treated group. This significance showed that histological changes associated with remodelling have been generated successfully in an animal asthma model.
In airway remodelling, one of the most important factors determining tonus is the smooth muscle of the airway. In patients with asthma, smooth muscle cells in the airway increase in number as well as in volume.15 Koumondouros et al. studied the relationship between micro environment and the airway in sheep and found smooth muscle hypertrophy and increased vascularisation in animals that were in constant contact with house dust mites. These changes were primarily seen in the lower and middle airways and were associated with disease in the small airways.16 In experimental models with statins, two current studies with simvastatin have mentioned its effects particularly on remodelling of the airways. A study by Shaafsma et al. investigated the changes occurring with mevalonate cascade blockage in the rebuilding of the airway by observing the changes in airway tissue of asthma-induced mice that were given simvastatin. Considering the hypothesis that the fibroproliferative balance occurring in the airway results from the increased deposit of the extracellular matrix, a significant decrease in the levels of TNF-alpha and IL-1, which affect the secretion of tumour growth factor-beta 1 (TGF-β1) that triggers this change, was observed with simvastatin. This effect was reported to be due to the blockage of RhoA by simvastatin through blockage of intracellular posttranslational signalling molecules.17 In our study, we histologically measured the subepithelial muscle thickness which was found to be significantly less in the atorvastatin group than in the placebo group. This result demonstrates the inhibitory effect of atorvastatin on smooth muscle hyperplasia.
Besides the histological changes, asthma also induces a chronic inflammation in the airways. The cytokines mainly held responsible for the triggering and continuation of inflammation have been IL-4, IL-5 and IL-13; although recent studies with TNF-alpha have demonstrated striking results.18 Har Seong Nam et al. studied the biological effect of TNF-alpha in animal models and found that the blockage of TNF-alpha resulted in the inhibition of IL-4, IL-5 and IL-6.19 The anti-inflammatory effect of statins on a cellular level has been demonstrated in various studies.20 By inhibiting LFA-1 and MHCII in antigen presenting macrophages and endothelial cells, the statins hinder extravasation of leucocytes, inhibit NK activity and decrease the levels of TNF-alpha, IL-1 and IL-6.21 Shyu et al. studied the effect of atorvastatin in tissue cultures and observed that atorvastatin caused decreases in the levels of TNF-alpha secreted by macrophages.22 In a similar, but later investigation by Awad and Sharif, the immunomodulatory effect of rosuvastatin was studied. The authors studied the tissues of mice with experimentally induced ischaemic reperfusion impairment, particularly the ischaemic liver as well as the heart, lung and kidney. In the mouse group receiving rosuvastatin for reperfusion damage, they showed that the levels of TNF-alpha, IL-6 and monocyte chemoattractant protein-1 (MCP-1) decreased while the levels of anti-inflammatory IL-10 increased.23
Apart from decreasing the plasma cholesterol level, statins also show an anti-inflammatory effect. Particularly cytokines IL-4 and IL-5 play very important roles in inflammation. Kim et al. demonstrated that simvastatin inhibited the synthesis of IL-4 and IL-5 in the lungs of mice induced with ovalbumin; they also found that statins decreased the specific IgE of ovalbumin as well.24 In another study, McKey et al. found that IL-4 and IL-5 secretion decreased in thoracic lymph node cultures of mice sensitised with ovalbumin and treated with intraperitoneal simvastatin along with decreases in IL-4 and IL-5 in BAL. In the same study, it was also shown that simvastatin therapy decreased eosinophiles and macrophages in BAL. In the presence of specific adhesion molecules and chemokines, the inflammatory cells migrate to the airways. Eosinophiles and macrophages secrete LFA-1, and simvastatin has been shown to be effective in this way.25
In their study on ovalbumin-sensitised BALB/c mice, Amir et al. found that the role of simvastatin in decreasing the inflammation in the airways was primarily related to mevalonate and that simvastatin decreased Th2 and TNF-alpha in BAL. Simvastatin also significantly decreased the levels of IL-4, IL-13 and TNF-α in lung tissue.26 In a later study, Amir et al. demonstrated that, mediated by IL-13, simvastatin also decreased goblet cell hyperplasia. In our study we also observed a decrease in goblet cell hyperplasia.27
IL-17 increases cytokine-chemokine secretion in human bronchial fibroblasts and smooth muscle cells in human airways, thus increasing allergic reactions in the airways. Imamura et al., in their study on ovalbumin-sensitised BALB/c mice treated with intraperitoneal pravastatin, showed that pravastatin inhibited sensitivity to OVA antigen by decreasing IL-17 secretion. The authors also found that statins decreased the serum IgE production as well as cytokine production by immunocytes in thoracic lymph nodes; thus statins by inhibiting cytokine inhibited airway inflammation in patients with bronchial asthma.28 In our study, when the levels of IL-4 and IL-5 in atorvastatin and saline receiving mice groups were compared, the levels were found to be significantly lower in atorvastatin-receiving mice.
Atorvastatin also has an effect on mast cell degranulation, which plays an important role in the pathogenesis of asthma. It has been shown that atorvastatin inhibits mast cell degranulation stimulated both immunologically and non-immunologically.29 It has also been reported that atorvastatin is effective on eosinophiles, cells with a significant role in late-phase allergic reactions and asthma pathogenesis, and inhibits eosinophile accumulation in BAL following allergic stimulus.30 In our study, when the atorvastatin receiving and placebo groups were histologically compared, there was a considerable decrease in the number of mast cells in the atorvastatin-receiving group.
Platelet-activating factor (PAF) is a phospholipid activator and pro-inflammatory mediator involved in the pathogenesis of asthma and other allergic diseases.31,32 In asthma patients, there is an increase in PAF receptors, and the blood PAF level rises particularly during asthma attacks.33,34 There have been many studies on antagonising this mediator in the treatment of asthma. Atorvastatin also demonstrates a PAF antagonist effect.35 Atorvastatin is a statin that inhibits many processes and mediators in the course of inflammation and remodelling such as mast cell degranulation, eosinophile accumulation, IL-5, IL-6, TNF-α and PAF. For this reason, atorvastatin has been considered to be more effective than PAF-antagonists alone in the treatment of asthma.
Clinical trials on adults have shown statins to be effective in asthma. Cowan et al. studied the effect of simvastatin on the regression of symptoms and required steroid dose in asthma patients on inhaled steroid therapy. 40mg of Simvastatin was added to the treatment regimen of 51 patients on fluticasone and gradually the inhaled steroid dose was reduced. The addition of statin to the therapy resulted in lower doses of fluticasone being required.36
Our study was not without limitations. For example, the positive outcomes identified in animal studies are not possible to adapt the work directly to people. Histological changes in the application of the corrective effect of atorvastatin in asthmatic airways also available to determine whether or not a continuous follow-up period are not included in this work plan.
In conclusion, the current study has shown that administration of atorvastatin is effective in resolving the established chronic histopathological changes of lungs in a murine model of asthma. Further studies with long-term treatments which evaluate the effects of atorvastatin on lung inflammation and remodelling are needed.
Ethical disclosuresConfidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Protection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
Conflict of interestThe authors have no conflict of interest to declare.