INTRODUCTION
The efficacy of subcutaneous immunotherapy in individuals allergic to inhalant allergens is well documented. However, systemic reactions are possible, and the medical management of each single injective procedure over the few years duration of this treatment is quite complex and requires access to resuscitative measures. Sublingual immunotherapy (SLIT) has been indicated as a safer and simpler approach, and several studies have showed remarkable clinical efficacy4-11. On this basis, the European Academy of Allergy and Clinical Immunology (EAACI) as well as the World Health Organization (WHO) published position papers on sublingual allergen immunotherapy3,12 where this therapeutical approach was suggested as a valid alternative to the traditional subcutaneous route. In the following years several trials were performed involving patients suffering both seasonal and perennial rhinitis. Most studies provided clear-cut evidence of efficacy6-10,13-20 while a few others only provided evidence for a favorable trend in several clinical parameters21-24. In all cases the safety of the sublingual route was confirmed.
Patients' compliance to SLIT is a relevant aspect for this therapy, which has to be continued for a few years. In particular, reaching the maintenance dose with daily administration of increasing doses in adult and pediatric patients might be particularly challenging. In fact, the pre-seasonal induction schedules of SLIT in patients allergic to pollens are usually performed during the late winter. In the non-infrequent cases a cold, a flue, or a minor upper airways disease might occur, this therapy is often interrupted and started over. On this basis rush induction regimens have been used, which cut the conservative 20 to 30 day induction phase down to a week or less9,14,17,18,21. Direct comparison between different rush schedules needs dedicated studies. Recently, a report compared the safety of three dosage regimen and indicated very few and minor side effects with any protocol25. Here, we evaluated in parallel the efficacy and the safety of sublingual immunoterapy initiated with an 8-day, 15-day or 20-day induction protocol, respectively, in patients with sensitization to seasonal and perennial inhalant allergens, over a 2-year follow up period.
MATERIAL AND METHODS
Patients
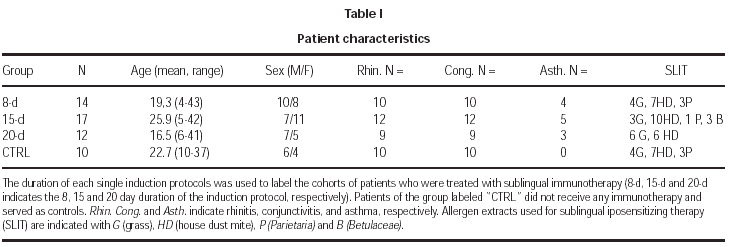
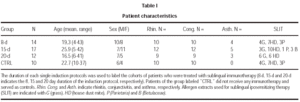
Fifty-three patients were recruited at the same outpatient clinic. They all gave a history of seasonal allergic rhino conjunctivitis of at least 2 years duration and positive skin-prick test to one or more of the following allergen extracts: house dust mites mixture (Dermatophagoides pteronyssinus and farinae), Ambrosia artemifolia, grass mixture (Dactlylis glomerata, Festuca pratensis, Lolium perenne, Phleum pratense, Poa pratensis), Parietaria judaica and Betulaceae mixture (Betula verucosa, Corylus avellana, Alnus glutinosa) (ALK-Abellò S.p.A., Milan, Italy). Each allergen was purified and biologically standardized, as previously described7. The skin prick test was performed according to the recommendations of the European Academy of Allergy and Clinical Immunology26 and positive results were expressed with a score ranging from 1 to 4, accordingly. Patients with mild asthma were included whose baseline Forced Expiratory Volume in one second (FEV1) at the first visit was above 75 percent of the predicted. Asthmatic patients fulfilled the criteria for classification of intermittent or mild persistent asthma, according to the International Guidelines for the Diagnosis and Treatment of Asthma (GINA) (National Institutes of Health, NIH publication number 02-3659, revised 2002).
Study design
This was an open study, where consecutive patients were randomly assigned to any of the following 4 groups: controls (untreated), 8-day induction, 15-day induction, and 20-day induction. Patients assigned to each group were matched for age, sex, and number of sensitizations (tables I and II).
Immunotherapy protocols
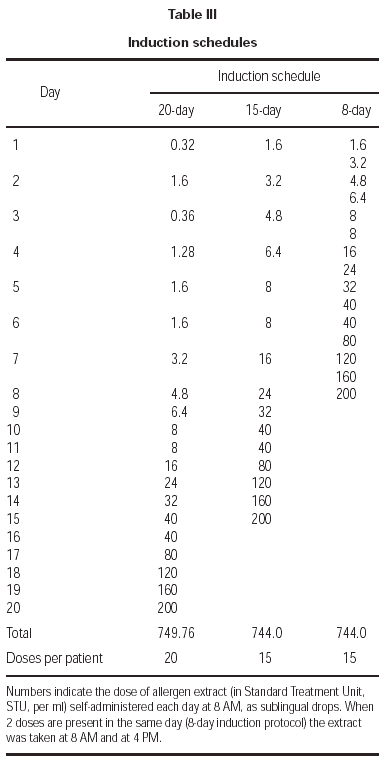
The treatments used in the study were glycerin/ phenol solutions prepared from biologically standardized aqueous extracts27, whose allergen content in major allergens was expressed in Standard Treatment Units (STU) per ml (ALK-Abellò).
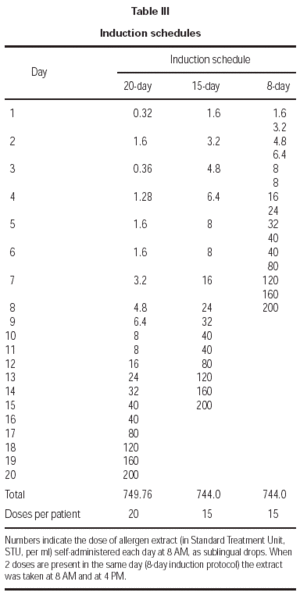
The duration of the treatment was 2 years. Four different concentrations of each extract were sequentially used, namely 8 STU, 40 STU, 200 STU, 1000 STU per ml. This allowed for each induction protocol a daily or twice-a-day administration schedule. In table III the amount of allergen which was self-administered per dose and per day in each group of patients is shown; for the 20-day induction protocol this corresponded to a progressive daily increment from 1 to 5 drop for each of the 4 concentrations; for the 15-day protocol the same schedule was followed, but starting with the 40 STU/ml concentration; for the 8-day induction protocol the same progression schedule as in the 15-day protocol was applied, but with a twice-a-day administration. At maintenance, 1000 STU of each extract were taken daily. For each allergen immunotherapy, the treatment was initiated at January 1, 1999, and continued for 2 years. This corresponded to the administration of the following cumulative amounts of major allergens: 115.2 μg Der p 1 plus 57.6 μg Der p 2 (house dust mite mixture), 72 μg group V grass allergens (grass mixture), 648 μg Bet v 1 (Betulaceae), 16.8 μg Par J 1 (Parietaria), per year, respectively. Cumulative doses were administered which were roughly three times higher than those administered with corresponding protocols of subcutaneous immunotherapy.
Symptom and medication scores
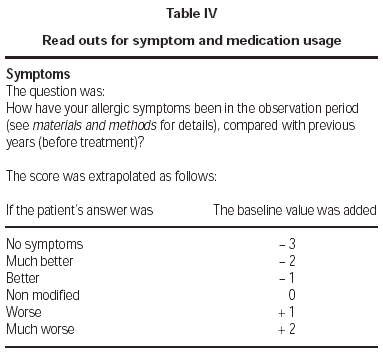
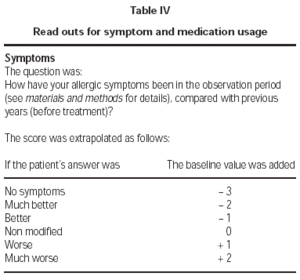
An arbitrary value of 5 was attributed to the baseline symptom and medication usage cumulative score (i.e., before SLIT). This was modified according to the answers that patients gave to questions separately concerning symptom and medication usage, as indicated in table IV.
Table IV Read outs for symptom and medication usage
In the case of sensitization to the perennial allergen, patients were asked to make an overall symptom and medication assessment referring to the previous 3 months, at each visit. The mean value of the first and of the second group of 4 trimesters was used as the read-out for the 1-year and 2-year symptom score, respectively.
In the case of sensitization to grass, Betulaceae, and Parietaria, the evaluation referred to the overall symptom assessment during the season when pollen counts were peaking in the Northern Italy area where patients were living. This corresponded to February through May for birch, March through June for grass and April through September for Parietaria, respectively. The first and second year evaluation were done at the first visit following the corresponding pollination season, respectively.
Spirometric evaluation
All patients included in the study performed spirometry at baseline (t = 0) and yearly thereafter. FEV1 was measured using a Spirolab II (Med Electronics, Inc., Baltimore, MD) and expressed as percent of predicted value according to Quanjer28.
Adverse event recording
During the induction phase, any local or systemic symptom occurring within 1 hour were recorded under supervision and documented at study site. Patients were asked to report any delayed (i.e., within 48 hours) local or generalized symptom occurring during the maintenance phase of treatment to the study investigators.
Statistical analysis
Symptom and medication scores and skin-prick test scores were compared using the two-tailed Mann-Whitney U-test for non-parametric data. FEV1 values were compared using the two-tailed t-test for paired data. Calculations were performed using the InStat-3 Software (Graphpad Software Inc, San Diego, CA). P-values less than 0.05 were considered to be statistically significant.
RESULTS
Clinical parameters
Symptom and medication usage
All patients completed the study. In the case of seasonal sensitizations, both for patients receiving SLIT and for untreated controls the presence of symptoms and the usage of medications closely paralleled the counts for the corresponding pollens in Northern Italy (not shown).
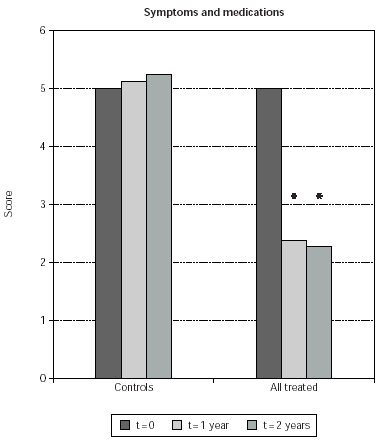
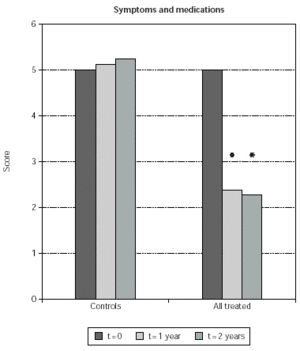
Symptom and medication usage scores in the immunotherapy groups, when cumulatively considered, were 48 and 50 % reduced, at 1 and 2 years since beginning of SLIT, respectively (p < 0.0001 in both cases) (fig. 1). At difference, values of symptom and medication usage were unchanged in the control, untreated group (fig. 1).
Figure 1.--Symptom and medication usage scores are shown on the y-axis. The baseline value was arbitrarily set to 5, and modified at subsequent clinical controls as indicated in Table IV. Scores obtained from control patients (controls) and from SLIT treated patients, cumulatively considered (all treated), are shown. Scores were measured at baseline (t = 0) at the times indicated in the legend. The asterisk indicate significant p values for the indicated data versus time 0 and correspond to p < 0.0001.
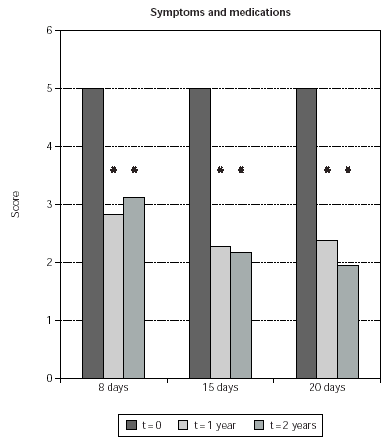
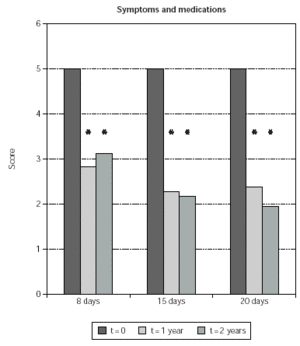
Symptom and medication usage reduction was significant also when comparing time-matched scores of SLIT treated patients with untreated controls at 1 and 2 years (51 % and 55 % less symptoms; p < 0.0001 in both cases) (fig. 1). Symptom and medication usage reduction at 1 and 2 years was significant also when patients treated with each induction protocol were separately considered (p ≤0.0005) (fig. 2). No significant difference was observed when comparing time-matched scores observed with the different induction protocols (fig. 2).
Figure 2.--Mean values of symptom and medication usage scores from SLIT treated patients are shown on the y-axis. Patients are grouped according to the induction protocol, which they were assigned to (shown on the χ axis). The times at which the results were obtained are indicated in the legend. The asterisk indicate significant p values for the indicated data versus time 0 and correspond to p < 0.0001.
Side effects
No immediate (within 1 hour) or delayed systemic reactions, or severe local reactions were observed during either the induction phase or the maintenance phase of the treatment. During the induction phase, 705 doses were cumulatively self-administered by the 43 patients in the 3 protocols. One patient belonging to the 15-day induction group suffered nose itching and sneezing as an early local effect after each of the first 15 doses (2.1 %). He did not require treatment or discontinuation of SLIT. No side effects were reported in the maintenance phase of any treated group, when a total of 2480 doses were self-administered.
Objective test
FEV1 values
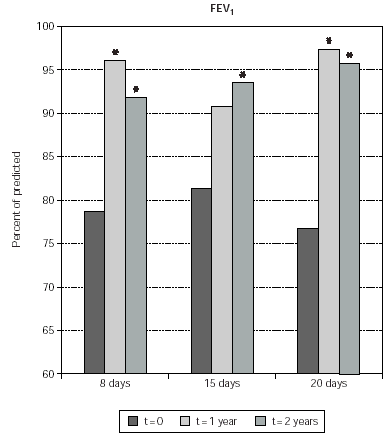
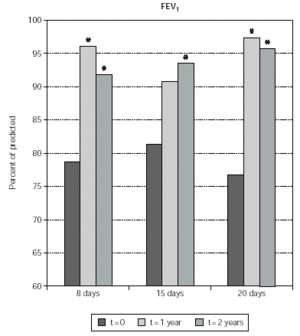
Asthmatic patients who underwent SLIT significantly improved FEV1 values after 2 years of therapy with any induction protocol (fig. 3). In particular the predicted value of FEV1 was 14.8, 12.9 and 18.8 % improved at 2 years in the 8-day, 15-day and 20 day induction protocol, respectively. Results were significant also when comparing FEV1 absolute values (not shown). In the 8-day and 20-day induction protocol a significant FEV1 improvement was already observed after 1 year of treatment (fig. 3).
Figure 3.--The Forced Expiratory Volume in one second (FEV1) is shown on the y-axis. Values are expressed as percent of predicted. Patients are grouped according to the induction protocol, which they were assigned to (shown on the x axis). The times at which the results were obtained are indicated in the legend. The asterisk indicate significant p values for the indicated data versus time 0 and correspond to p < 0.05.
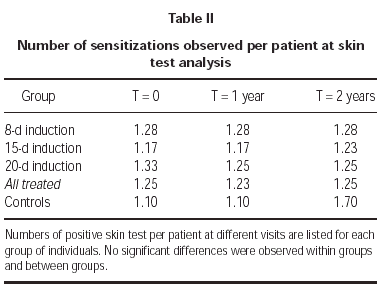
Skin tests
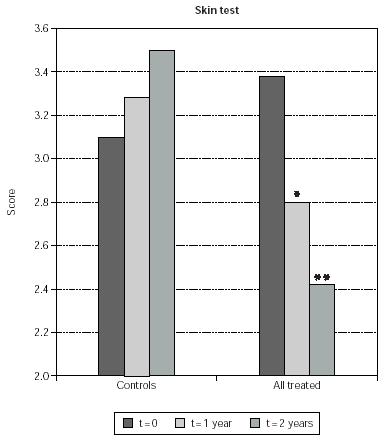
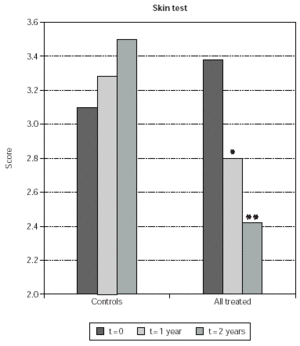
At baseline no difference was observed in skin test scores for the major sensitizing allergens when comparing control patients with those who were selected for treatment with immunotherapy (fig. 4). At 1 and 2 years since the beginning of the study, the scores of the early skin response to allergens used for SLIT in treated patients were 18 % and 24 % reduced respectively (p < 0.0001 in both cases) (fig. 4). At difference, no significant changes were observed in the size of the early skin response to major sensitizing allergens in control, untreated patients (fig. 4). Reduction of skin-test scores was significant also when comparing time-matched scores of SLIT treated patients with untreated controls at 1 and 2 years (14 % and 30 %; p = 0.0123 and p < 0.0001, respectively) (fig. 4).
Figure 4.--Mean values of skin test results are shown on the y-axis. They were calculated as indicated in Material and Methods. Mean values obtained from control patients (controls) and from SLIT treated patients, cumulatively considered (all treated), are shown. The times at which skin test were performed are indicated in the legend. The asterisks indicate significant p values for the indicated data versus time 0 and correspond to p = 0.0123 (*) and p < 0.0001 (**).
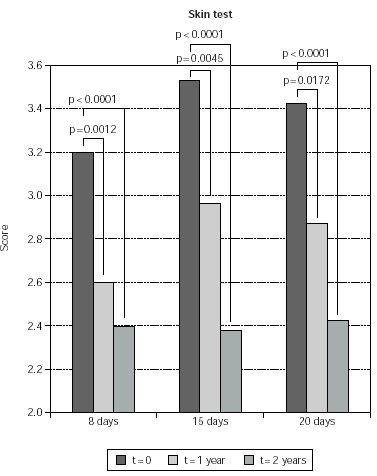
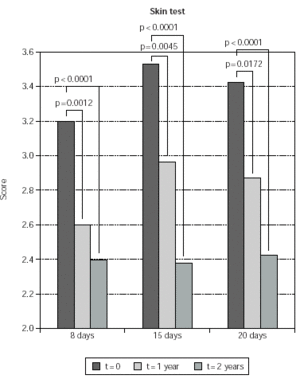
Reduction of the skin test scores was significant both at 1 and at 2 year also when considering singularly the groups treated with the different induction protocols. Namely, in the 8-day treated group, reduction of skin scores was 18 % and 24 % at 1 and 2 years, respectively. In the 15-day treated group, reduction of skin test scores was 15 % and 31 % at 1 and 2 years, respectively. In the 20-day treated group, reduction of skin test scores was 15 % and 22 % at 1 and 2 years, respectively (fig. 5). No significant difference was observed when comparing skin test results observed in the 8-day group at 2 versus 1 year, while a further 19 % and 13 % reduction of skin test scores (p < 0.0001 and p = 0.0041) was observed at 2, as compared to 1 year in the 15-day and in the 20-day induction group, respectively (fig. 5).
Figure 5.--Mean values of skin test results are shown on the y-axis. They were calculated as indicated in Material and Methods. Patients are grouped according to the induction protocol, which they were assigned to (shown on the χ axis). The times at which skin test were performed are indicated in the legend; p values for compared data are shown.
No significant differences were observed when comparing skin test scores at time matched visits since the beginning of study enter, in patients belonging to the different induction protocols. The only exception were patients belonging to the 8-day induction group, whose skin test scores at 1 years were 12 % lower than corresponding skin test values of patients belonging to the 15-day induction group (p = 0.0044).
DISCUSSION
Sublingual immunotherapy (SLIT) of allergic diseases has been indicated as a valid alternative to subcutaneous immunotherapy3,12. SLIT is easy to handle for patients, since it can be self-administered at home, and requires only periodical supervision by the allergologist. However, approved protocols require a quite complex induction regimen, which is pre-seasonal in the case of pollen allergies. During induction, different doses are to be taken daily for 20 to 30 days, thus requiring a certain degree of compliance until maintenance is reached and doses become constant. Would an upper respiratory disease or a flue occur, the protocol is stopped and restarted later on. This requires a further effort to complete the treatment. On this basis, rush induction protocols have been used, particularly in pediatric patients, which demonstrated clinical efficacy and good safety9,14,17,18,21. Recently, a direct comparison of different induction protocols was published, which showed the safety of shorter induction regimens25.
Here, we made an open study on SLIT in patients suffering rhinitis, conjunctivitis, and asthma and sensitized to common inhalant allergens. We compared efficacy of three induction protocols (8-day, 15-day and 20-day), which had different duration and/or different starting doses. The evaluation of side effects was included in the clinical follow up of these patients. We found that SLIT induced a highly significant reduction of symptom and of medication usage at 1 and 2 years. Untreated control patients remained clinically stable. No differences were found when comparing the improvement of these clinical parameters obtained with each induction protocols. Patients included in this study were virtually monosensitized, and this could have had a role in the remarkable clinical improvement we observed. Non systemic side effects were recorded, and only mild disturbances occurred in a single patient during the 15-day induction regimen. The observed frequency of side effects, and the kind of adverse reactions were in accordance with published data13,29. Taken together these clinical results indicate that SLIT with the 8-day induction regimen is as safe and as efficacious as SLIT with longer induction protocols.
The oral cavity is an immunologically privileged site. In fact, it has been long known that in animal models the outcome of the exposure to antigen via the gastro enteric route is different if ingestion rather than sublingual exposure is used30. The underling immunological mechanisms likely involve dendritic-like cells (Langheran cells), which have been identified in the oral mucosa31. These cells may produce lymphokines, such as IL-12 or TGF-β, which drive the T cell response towards a Th1 or a regulatory phenotype, respectively. Indeed, it has been recently demonstrated in a mouse model that lipopeptides can be taken up preferentially by dendritic cells within the oral mucosa, and promote an immune response characterized by high level of IFN-γ and IgG2a production32. In principle, on this basis it might be explained how the administration of allergen via the sublingual (but not the oral)12 route can result in an effective treatment of respiratory allergy, and possibly of atopic dermatitis33. In particular, the recirculation of allergen specific T lymphocytes at peripheral lymphoid and non-lymphoid organs34, including the oral mucosa, might put SLIT in action, e.g. via the modulation of chemochine receptors36. Experimental evidence for these mechanisms awaits dedicated studies.
Here, we show that the baseline levels of skin sensitization to sensitizing allergens were significantly reduced at 1 and 2 year from beginning of SLIT, in accordance with previous reports6,15,19,29. In control patients skin reactivity remained unchanged. Moreover, FEV1 values were significantly increased at 1 and 2 years as compared to baseline in asthmatic patients. This result may be explained with the reduction in the lower respiratory tract of the allergic inflammation, which is casually associated with broncho constriction in asthmatic patients37. No differences were observed when comparing reduction of skin sensitization and improvement of FEV1 obtained with the different induction protocols. Taken together, these objective results indicate that an active allergen specific immunomodulation took place following SLIT, which affected both the immediate skin reaction to allergen as well as the inflammation at the target organ in allergic asthmatic patients.
In conclusion, SLIT is a clinically effective and safe immunomodulating therapy for allergic patients affected by respiratory allergies. Compliance to SLIT may be improved by reducing the induction regimen to 8-day, with no losses in efficacy and safety.