INTRODUCTION
Heparin is an anticoagulant drug discovered by Mc Lean in 19161, even though it was in 1938 when it was used for the first time2. Since then, it has been used very frequently as prevention and treatment of thromboembolism.
Firstly, the only existing heparin was the sodium one, which required intravenous administration, and only in the first 70s is the calcium heparin discovered, that allows its subcutaneous administration and its use as prophylactic treatment.
Non fractionated heparin (NFH) is a glycosaminoglycan of heterogeneous molecular weight (mean 15000 daltons) that is obtained and purified from the bovine lung or the intestinal mucosa of the pig. It can be administered by intravenous or subcutaneous via (intramuscular via must be avoided), and it is commercialised in two forms, as sodium salt (iv) or as calcium heparinate (sc).
In 1980, by means of fraction techniques (enzymatic, chemical or physicochemical), purification and synthesis, more homogeneous preparations are obtained from low molecular weight polymers (mean weight 5000 daltons), denominated fractionated or low molecular weight heparins (LMWH), that alike the NFH potentiate the inhibitor effect of the antithrombine III. They present some advantages compared to NFH, such as a greater availability, longer semi-life, and a more efficient and safer antithrombotic effect.
The following belong to that group:
Bemiparin (Hibor®).
Dalteparin (Boxol®, Fragmin®).
Enoxaparin (Clexane®, Decipar®).
Nadroparin (Fraxiparina®).
Tinzaparin (Innohep®).
Both the non fractionated heparins and the low molecular weight ones, although these last ones not as frequently, produce adverse reactions amply studied such as hemorrhage or osteoporosis when administered for longer than six months, and thrombocytopenia.
Nevertheless, there are some adverse reactions not as frequent, such as the allergic reactions. These have been known since 1950 but their diagnosis is still a challenge due to the polytreatment often followed by the patients, the scarce reliability of the in vivo diagnostic techniques, and the lack of safe in vitro tests that could support the diagnosis and avoid risks to the patients with anaphylactic reaction.
In this work, we describe two patients whose allergy to heparin was proven by a new in vitro diagnostic technique: the basophil activation test (BAT). The usefulness of this technique in the in vitro diagnosis of drug allergy and other allergens has been previously confirmed3-5.
PATIENTS AND METHODS
Case report 1
18 year old woman, with background of multiple surgeries due to congenital anomaly of short femur. During the last post-surgery and after eight days of treatment with subcutaneous enoxaparin and metamizol, two hours after the last dose of subcutaneous enoxaparin the patient presents generalised pruritic erythematous infiltrated plaques and palpebral angioedema. She went to the Emergency Service, where she was administered intravenous corticosteroids and antihistamines, presenting a good response.
After consulting with the Orthopedic Surgery Department, the patient was indicated to immediately stop the administration of heparin. In the anamnesis, no administration of any other drug in the previous six hours or any other triggering factor was referred by the patient, who tolerated metamizol subsequently.
Case report 2
Twenty-seven year old woman, with personal background of seasonal allergic rhinoconjunctivitis with sensitisation to grass pollen, under treatment with subcutaneous enoxaparin after surgery of disk hernia. The patient came to consultation referring, on the seventh day under treatment with enoxaparin, itching in popliteal area, buttocks and scalp. She continued treatment until six days later, when approximately 15 minutes after administration of enoxaparin, she started with dizziness and sweating that lasted one hour and three hours later, erythematous infiltrated plaques in upper limbs, back and anterior part of the tights. The patient did not present any other symptoms, and had a good evolution once the treatment was stopped, requiring no medication.
After a recovering period going from three to four weeks, the patients underwent the following in vivo allergological study:
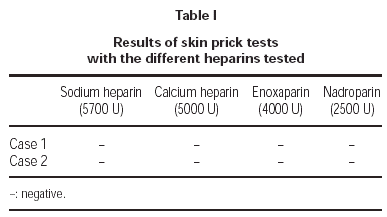
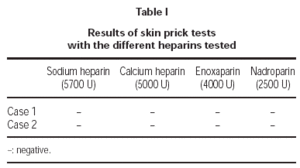
1. Skin Prick tests with NFH (sodium heparin and calcium heparin) and with LMWH (enoxaparin and nadroparin).
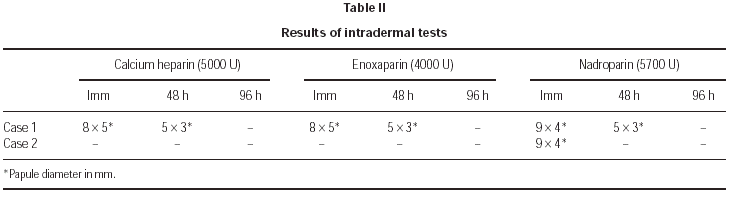
2. Intradermal tests with NFH (calcium heparin) and LMWH (enoxaparin and nadroparin).
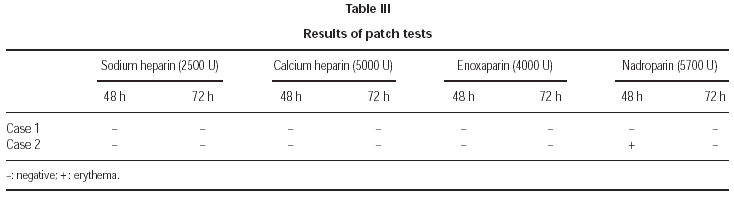
3. Patch tests with NFH (sodium heparin and calcium heparin), and with LMWH (enoxaparin and nadroparin).
Blood test and erythrocyte sedimentation rate was also performed.
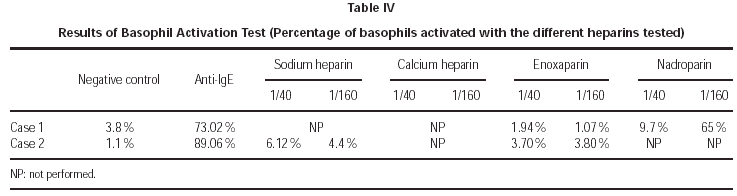
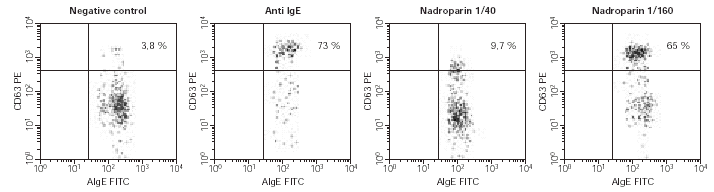
Concerning the in vitro allergologic study, the following tests were performed: Total serum IgE and Basophil Activation Test (BAT). This test was performed following the technique previously described3,4. After in vitro allergen-specific stimulation, basophils become activated and express CD63. This can be detected by flow cytometry using monoclonal antibodies, like anti-CD63-PE and anti IgE-FITC. In the first case nadroparin was used at two dilutions (1/40 and 1/160), and in the second case calcium heparin and enoxaparin was used at the same concentrations.
RESULTS
In the allergologic study performed, we found (tables I-IV):
1. Prick tests with sodium and calcium heparin, enoxaparin and nadroparin, were negative in both cases.
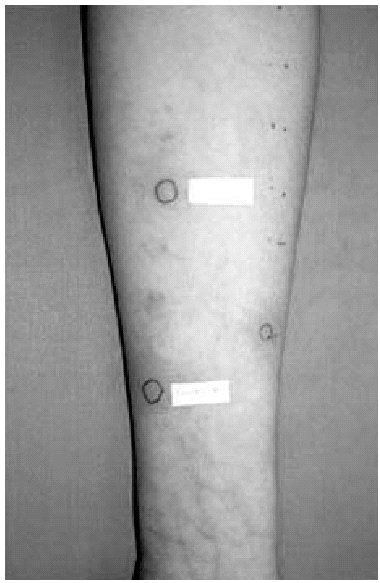
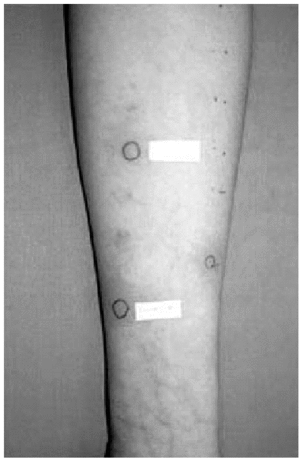
2. Intradermal skin tests with immediate lecture, at 48 and at 96 hours, with calcium heparin, enoxaparin and nadroparin, were positive in the immediate lecture and after 48 hours for the first patient. In the second case, we only obtained a positivity to nadroparin in the immediate lecture (fig. 1).
Figure 1.--Intradermal tests: positive result to Nadroparin in the immediate lecture.
3. The same drugs were also tested by patch tests, with lecture after 48 and 72 hours, finding only a positivity after 48 hours to nadroparin in the second patient.
4. Concerning the in vitro study, total serum IgE was within normal values (60.9 kU/l and 29.8 kU/l respectively).
5. Basophil Activation Test was performed with nadroparin and enoxaparin in the first case, being positive to enoxaparin, and with sodium heparin and enoxaparin in the second case, being positive for sodium heparin (fig. 2).
Figure 2.--Example of positive case in BAT. In the upper right square the activated basophils expressing CD63 in basal determination, in response to anti IgE (positive control) and in response to 1/40 and 1/160 Nadroparin concentrations.
DISCUSSION
The first description of a case of allergy to heparin was done by Chernoff in 19516. From that moment, there are several works in the literature, such as the one by Serradimigni in 19687 that describes three cases of allergy to heparin, and the one by Curry in 19736. Subsequently, more cases have been published, generally only one case is described in each publication, because this is a very rare pathology.
In the literature, we can find hypersensitivity reactions to heparin type I, II, III and IV according to the classification by Gell and Coombs8, even though the type IV is the most frequent and documented one.
Concerning the type I or immediate hypersensitivity reactions, in 1975 Dukes described a case of allergic conjunctivitis as reaction to non fractionated heparin. In 1977 Savas et al9 described an elderly woman who developed severe bronchospasm three hours after starting treatment with intravenous heparin.
The cases of anaphylactic shock are very rare. The first one was described by Coon and Willis in 196610. It is not possible to anticipate the patients who could develop a shock, although it is postulated that patients with localised hemorrhagic necrosis due to heparin, could trigger anaphylactic reactions.
Regarding the type II or cytotoxic reactions, in a study performed by Harenberg et al11, the association between thrombocytopenia, thombosis and heparin induced antibodies (IgG) was observed in 3 % of the patients who followed post-surgery treatment. They also observed that one-fourth of patients with skin reaction triggered by heparin and with IgG antibodies developed complications such as lung thromboembolism or skin necrosis. This is why the authors advise that skin test to this drug should be performed to those patients whose delayed skin reaction could be heparin-induced, and eliminate the drug in the future if the study was positive.
As for type III reactions, the vasculitis have been described12. Lefebvre et al13 described an aged woman who developed skin necrosis areas after administration of subcutaneous heparin on the lipodystrophic abdominal area due to insulin subcutaneous injections, showing the biopsy a leukocytoclastic vasculitis.
The type IV or delayed reactions are the most prevalent and documented in the literature, being more frequent in adult women. The reactions usually take place 2 to 4 days after heparin administration. Among the skin reactions, persistent erithema, urticaria and/or exfoliative dermatitis are included. Up to now, the performance of intradermal or patch tests was considered important for this group of patients in order to confirm the etiological agent, since there was no other kind of test for their in vitro diagnosis.
Several studies in the literature state that skin tests confirm and detect cross reactivity between NFH, LMWH and heparinoides, being the intradermal tests the most reliable diagnostic tool14, even though the sensitivity and the specificity of these tests is yet to be determined.
In some cases, provocation tests must be performed in order to reach a final diagnosis15, in spite of being this kind of tests quite risky for the patient. On the light of our results, we suggest using this new in vitro diagnostic technique, the Basophil Activation Test (BAT), in the study of possible sensitisation to heparins. In some cases of shock, it is advisable to perform the BAT before the skin test, in order to avoid the risk it bears for the patient, and always before the provocation tests.