Drug allergies are reactions within the context of drug hypersensitivity reactions, which are caused by immunological mechanisms due to a previously sensitising drug. Beta-lactam antibiotics (BLA) are the leading agents causing drug hypersensitivity reactions in children.
The aim of this study is to evaluate the diagnostic importance of in vivo and in vitro diagnostic tests in children with suspected immediate-type BLA hypersensitivity and to investigate the frequency of their use for the final diagnosis.
MethodsPatients admitted to the Outpatient Clinic of Division of Paediatric Allergy and Immunology with suspicion of immediate-type BLA hypersensitivity between December 2014 and December 2018 were investigated. Patients with a history of immediate reactions to BLA were examined by performing drug specific IgE, skin prick tests, intradermal tests and drug provocation tests (DPT).
ResultsDuring the study period, 148 patients were admitted to our clinic with suspected immediate-type BLA hypersensitivity. Forty-eight patients completed all assessment steps and were enrolled in the study. It has been shown that 27 patients did not have drug allergy. BLA hypersensitivity was proven in 21 patients by using in vivo test algorithm. More than half of the patients were diagnosed via skin tests with culprit drug.
ConclusionAllergy work-up should be performed in patients with immediate reactions to BLA. A skin test can demonstrate BLA hypersensitivity in most patients. Thus, skin tests should be performed prior to the drug provocation test.
Drug allergies are reactions within the context of drug hypersensitivity reactions, which are caused by immunological mechanisms due to the previously sensitising drug.1 These reactions are classified as immediate and non-immediate reactions, based on the time when the symptoms are observed following exposure to the drug. Immediate reactions occur within the first hour after drug administration, while delayed reactions occur at least 1–2h after drug administration.2,3 Immediate reactions are urticaria, angio-oedema, conjunctivitis, rhinitis, bronchospasm, gastrointestinal symptoms and anaphylaxis. On the other hand, delayed reactions include maculopapular rashes and delayed urticaria.
As with all drug hypersensitivity reactions, the major problem with drug allergies is inadequate diagnosis or overdiagnosis. Amongst children, maculopapular rashes are frequently observed during viral infections and these rashes can be defined as drug allergy by families and physicians because administration of drugs is common during infections. Such misdiagnoses prevent necessary use of the correct drug and lead to the use of more expensive and less effective drugs. On the contrary, inadequate diagnoses may result in severe reactions and complications related to drug allergy.4,5
Beta-lactam antibiotics (BLA) are the leading agents causing drug hypersensitivity reactions. Although many patients stated that they had allergies against BLA, it has been shown that most of the patients did not have allergies to the suspicious drugs.6–8 Therefore, the diagnostic algorithm for BLA hypersensitivity should be fully implemented. According to the international guidelines, it is recommended that the examination and evaluation for drug allergy should be performed 4–6 weeks after complete regression of symptoms. However, this process may vary depending on the type and severity of the initial reaction.3 In order to prevent severe immediate reactions in patients with suspected BLA hypersensitivity, it is recommended to perform in vitro (penicilloyl G and V specific IgE) and in vivo tests (skin prick test, intradermal test) and when the result is negative, to perform an oral drug provocation test (DPT). In recent years, DPTs have started to be performed under safer conditions with the use of the penicillin kit, which enables skin tests to be performed under optimum conditions in children with suspected BLA hypersensitivity.
The aim of this study is to evaluate the diagnostic importance of in vivo and in vitro tests in children with suspected immediate-type BLA hypersensitivity and to investigate the frequency of their use for a final diagnosis.
Materials and methodsThis cross-sectional retrospective study was conducted by examining the files of patients who underwent diagnostic tests with suspected BLA hypersensitivity at the outpatient clinic of the Division of Paediatric Allergy and Immunology of Izmir Dr Behcet Uz Children's Education and Research Hospital. All data were taken from the medical records of patients. The data were anonymised by assigning a code to each patient. Only the principle investigator and the statistician had access to these data.
PatientsAll patients followed up with suspected BLA hypersensitivity in our outpatient clinic between December 2014 and December 2018 formed the base of the study. Reactions of BLA hypersensitivity were divided into immediate and non-immediate types based on time of drug intake and time to clinical symptoms in line with the recommendations of the European Network on Drug Allergy and the European Academy of Allergy and Clinical Immunology (ENDA/EAACI).1,2 Patients with immediate-type BLA hypersensitivity, i.e., who developed the reaction within the first hour after drug intake were included in the study. The ENDA/EAACI diagnostic algorithm was used in patients with suspected BLA hypersensitivity with immediate-type reaction. Patients who did not complete the diagnostic algorithm (those who did not come for a follow-up visit or testing of whom was not accepted by their parents) were excluded from the study. Demographic data (patient gender, patient age, age at the time of reaction, age at the time of testing, symptoms, name of the accused drug, time between drug intake and reaction, presence of additional atopic disease), clinical characteristics of patients, levels of specific IgE antibodies to penicilloyl G or penicilloyl V, skin tests (prick and intradermal) and oral provocation test results were recorded.
Data evaluationIn vitro testsIn vitro tests, which are specific IgE antibodies to penicilloyl G or penicilloyl V were studied by CAP-FEIA (Thermo Fisher Scientific, Upsala, Sweden). Results of ≥0.35 kUA/l were considered positive.
In vivo testsSkin Prick test and Intradermal testSkin prick test was performed with major determinant penicilloyl-polylysine (PPL) (5×10−5M) and minor determinant mixture (MDM) (2×10−2M) (Diater, Madrid, Spain), Amoxicillin-clavulonate and responsible antibiotic. Histamine (10mg/ml) was used for the positive control and saline for the negative control. The test was performed on the forearm of the patients and was read after 20min. Prick test was performed; if the prick test was negative, intradermal test was performed. The skin prick test was considered positive if there was an induration above 3mm along with a negative control with saline. Skin test prick test was performed according to ENDA/EAACI recommendations.1,6 The initial induration was measured for the intradermal test, and the test was considered positive if the change in the diameter of induration was >3mm when measured 20min after the first measurement.
Drug Provocation TestDPT was applied to patients who had no contraindication and negative results for skin prick test and intradermal test according to ENDA guidelines. The aim of the DPT was to confirm or exclude a reaction to the culprit drug.9 The doses were calculated by weight, as follows: amoxicillin 40mg/kg/day; amoxicillin/clavulanate, 40mg/kg/day; cefuroxime, 20mg/kg/day; cefdinir, 14mg/kg/day; penicillin V, 25mg/kg/day. Under observation in the hospital, the responsible drug was administered orally in divided doses (maximum five doses) at 30-min intervals until the full dose was reached. The test was terminated at the step in which the reaction was observed. Patients with a reaction were considered to have drug allergy and the culprit drug was banned. Patients who completed the test steps without any reaction were observed in the hospital for at least two hours after the last drug dose. DPT was performed with penicillin V, suspected beta-lactams (aminopenicillins, cephalosporins) and safe alternatives. In patients sensitive to aminopenicillins, DPT was performed with non-cross-reactive side chain cefuroxime. We used non-beta-lactam antibiotics as a safe alternative in cases presenting with anaphylaxis.
Statistical analysisData were analysed using SPSS statistical software, version 22 (SPSS Inc., Chicago, IL, USA). Frequencies and percentages were calculated. Age of study subjects was also defined using mean±standard deviation. The Chi-square test and Fisher's exact test were used for comparison between independent groups of categorical data. For all statistical tests, p<0.05 was considered statistically significant.
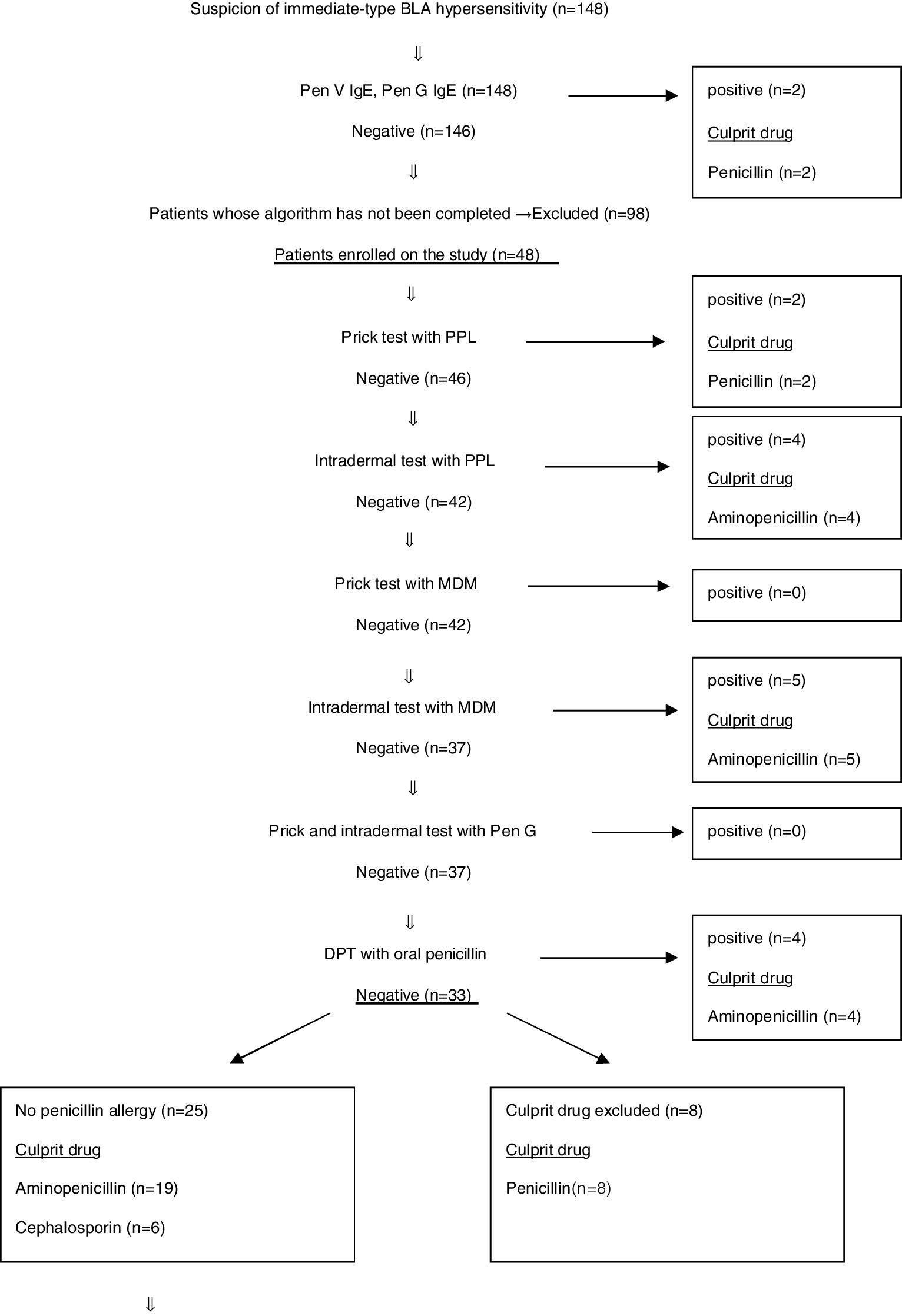
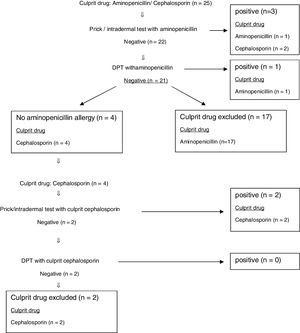
Results148 patients were admitted to our outpatient clinic with the suspicion of immediate-type BLA hypersensitivity between December 2014 and December 2018. Specific IgE antibodies to penicilloyl G or penicilloyl V were performed for all cases. Only two patients had positive results. Because of severe systemic reaction in these patients, there had been no necessity to use the in vivo test algorithm (Fig. 1) and they were considered to have allergy against BLA. Ninety-eight patients were excluded from the study because some parents did not approve in vivo tests, and some did not come to follow-up visits. Eventually, 48 patients were included in the study.
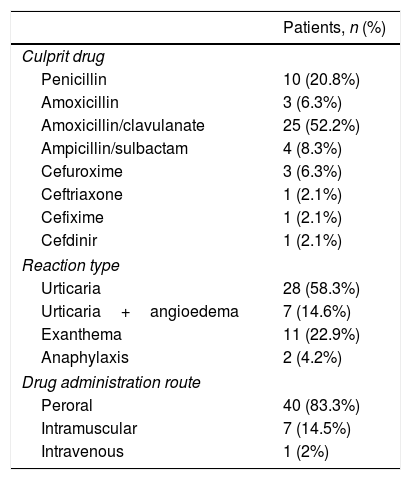
45.8% (n=22) of the patients included in the study were female. The mean age with standard deviation (SD) was 7.8±4 years, the average age at the time of reaction was 6.4±4.1 years and the mean age at the time of examination was 6.8±4.1 years (Table 1). The mean time from drug intake to allergy work-up was 8.3±4.2 months. Fifteen of 48 patients had atopic diseases. The distribution of diseases was as follows: atopic dermatitis 20% (n=3), food allergy 13.3% (n=2), allergic rhinitis 33.3% (n=5), and asthma 33.3% (n=5). Culprit drugs were penicillins and cephalosporins. The most frequently used culprit drugs were aminopenicillin group BLA. The most common clinical presentation was urticaria without angio-oedema 58.3% (n=28), and the second was non-specific rashes 22.9% (n=11). In two cases, anaphylaxis 4.2% (n=2) was observed within minutes after the drug administration. In both cases, the route of administration was intramuscular, and the drugs were ceftriaxone and cefuroxime.
83.3% of the reactions developed after oral intake. The distribution of suspected beta-lactam group drugs, reaction type and route of administration are shown in Table 2.
Distribution of the culprit drug, reaction type, and route of administration.
| Patients, n (%) | |
|---|---|
| Culprit drug | |
| Penicillin | 10 (20.8%) |
| Amoxicillin | 3 (6.3%) |
| Amoxicillin/clavulanate | 25 (52.2%) |
| Ampicillin/sulbactam | 4 (8.3%) |
| Cefuroxime | 3 (6.3%) |
| Ceftriaxone | 1 (2.1%) |
| Cefixime | 1 (2.1%) |
| Cefdinir | 1 (2.1%) |
| Reaction type | |
| Urticaria | 28 (58.3%) |
| Urticaria+angioedema | 7 (14.6%) |
| Exanthema | 11 (22.9%) |
| Anaphylaxis | 2 (4.2%) |
| Drug administration route | |
| Peroral | 40 (83.3%) |
| Intramuscular | 7 (14.5%) |
| Intravenous | 1 (2%) |
BLA hypersensitivity was proven in 21 (43.7%) patients by using in vivo test algorithm. It has been shown that 27 (56.3%) patients did not have culprit drug hypersensitivity. The in vivo test algorithm used for diagnosing suspected BLA hypersensitivity is demonstrated in Fig. 1.
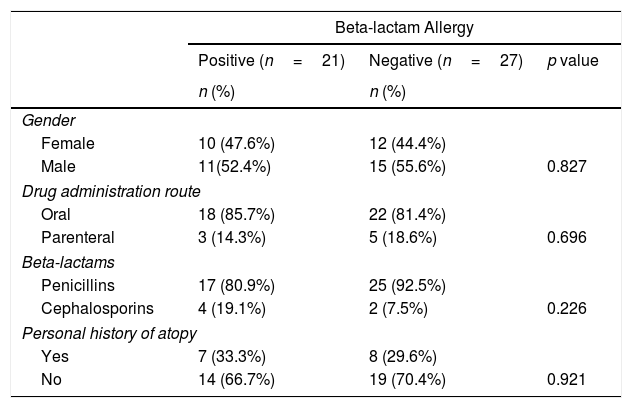
Forty-eight patients included in the study were evaluated and no significant difference was found between the patients with and without BLA hypersensitivity in terms of gender (Table 3).
Comparison of patient groups with and without beta-lactam allergy.
| Beta-lactam Allergy | |||
|---|---|---|---|
| Positive (n=21) | Negative (n=27) | p value | |
| n (%) | n (%) | ||
| Gender | |||
| Female | 10 (47.6%) | 12 (44.4%) | |
| Male | 11(52.4%) | 15 (55.6%) | 0.827 |
| Drug administration route | |||
| Oral | 18 (85.7%) | 22 (81.4%) | |
| Parenteral | 3 (14.3%) | 5 (18.6%) | 0.696 |
| Beta-lactams | |||
| Penicillins | 17 (80.9%) | 25 (92.5%) | |
| Cephalosporins | 4 (19.1%) | 2 (7.5%) | 0.226 |
| Personal history of atopy | |||
| Yes | 7 (33.3%) | 8 (29.6%) | |
| No | 14 (66.7%) | 19 (70.4%) | 0.921 |
The diagnosis of 73.3% of patients with penicillin allergy was confirmed by skin tests. Skin tests were negative in 26.7% of patients with penicillin allergy and sensitisation was demonstrated by DPT (Fig. 1).
Total of 32 cases were admitted with the suspicion of aminopenicillin allergy. It was shown that 17 of these patients had no allergies to penicillin or aminopenicillin by allergy work-up. Skin tests of 17 cases with PPL, MDM and aminopenicillins were negative. At the same time, DPT with both oral penicillin and oral aminopenicillin were negative in these patients. Therefore, the culprit drugs were excluded. The BLA hypersensitivity of 15 cases was demonstrated. Nine of these patients had positive skin tests with PPL and MDM. DPT was positive in four cases with oral penicillin. Thirteen cases with suspected aminopenicillin allergy had sensitisation to penicillin. Thus, these 13 patients proved to be allergic to both penicillin and aminopenicillin. It was also shown that two patients with aminopenicillin allergy tolerated penicillin. One of them had positive skin tests with ampicillin. The other one had positive DPT with oral ampicillin (Fig. 1). Non-beta-lactam antibiotics and cephalosporins which they had previously taken without reaction were determined to be safe for patients with aminopenicillin allergies. In order to determine an alternative antibiotic, patients who had positive amoxicillin–provocation test with clavulanic acid underwent provocation with cefuroxime, which has a non-cross-reactive side chain, and then a written information form including the list of prohibited and alternative antibiotics was given to patients. Five cases with aminopenicillin allergy were found to have used oral cefuroxime without problems more than once.
Six of the patients were admitted with suspicion of cephalosporin allergy. Four patients have tolerated DPT with penicillin and aminopenicillin. No reaction was observed. Sensitisation of two cases developing anaphylaxis (one with cefuroxime and other with ceftriaxone) has been demonstrated in the skin prick test.
DiscussionPenicillin is metabolised after intake. With metabolisation, 95% of penicillin is converted into the major antigenic determinants containing a penicilloyl core. The main major antigenic determinant used for the tests is penicilloyl polylysine (PPL). The remaining 5% is converted to minor antigenic determinants (or MDM, minor determinants mixture), such as peniciloate and penilloate.10 Therefore, skin tests with both PPL and MDM should be performed in the diagnosis of penicillin allergy. In accordance with the algorithm, we initially performed skin tests with PPL, then with MDM, because MDM is responsible for most of the serious allergic reactions such as anaphylaxis.11 Skin tests with ampicillin and amoxicillin, two other widely-used penicillin derivatives, can be performed by diluting commercial samples of these drugs to 1–20mg/ml.12,13 Systemic reactions were reported in 1.3% of all tests in literature.14 None of our patients had any adverse reactions during allergy work-up. Only two patients had Pen V-IgE positivity amongst 148 patients. Based on the results of the work-up, 21 of the 48 patients (43.7%) who completed the algorithm were proven to have sensitivity against the culprit drug. BLA sensitisation of 21 cases was confirmed via skin tests and DPT. It was found that 76.2% of the patients (16/21) had BLA hypersensitivity by using SPT and 23.8% (5/21) were diagnosed by using DPT. Misirlioglu et al.15 reported that in their study these ratios were 62.2% and 37.7%, respectively.
The optimal duration of DPT is still unknown.16 In our patients, we performed DPT with culprit drug at least six weeks from the reaction history and not exceeding 12 months. DPTs without skin testing can be considered in children with mild non-immediate BLA hypersensitivity reactions.16 However, DPT is contraindicated in life-threatening immediate reactions such as anaphylaxis. Therefore, those cases in which DPT is not feasible, skin tests are useful for revealing sensitivity. Sensitisation of two patients who had anaphylaxis history was demonstrated by skin prick test. DPT was not performed in these cases.
In our study, BLA hypersensitivity has been shown in 46% of the patients with isolated urticaria and in 85% of the patients with urticaria/angio-oedema. Gomes et al.9 reported that angio-oedema is associated with NSAID hypersensitivity. In our cases, BLA hypersensitivity was confirmed in the majority of patients with angio-oedema. Infections are one of the most important risk factors to consider, especially regarding reactions to antibiotics.9 Unnecessary antibiotic use in cases of viral infection will increase the number of admissions with suspicion of drug allergy. Most of the skin eruptions during BLA treatment in children are likely due to viral infections, which can mimic an allergic reaction.16 In the light of our study results, we can say that especially angio-oedema accompanying urticaria can be interpreted in favour of drug allergy.
According to our study, we can say that it is very important to assess whether a patient with aminopenicillin allergy is sensitive to penicillin.
Sensitisation of two cases developing anaphylaxis (one with cefuroxime and the other with cephalosporin) has been demonstrated in the skin prick test. In these patients, DPT and skin tests with penicillin and aminopenicillins were negative. This gives us the opportunity to tell the patients that they may use the penicillin or aminopenicillin group drugs safely. It has been shown that a drug provocation test (DPT) without previous skin tests in paediatric patients with non-severe delayed drug reactions is safe and effective.17 However, especially if there is a life-threatening reaction history, skin prick tests are still important in early mediated reactions. Additionally, Azevedo et al.18 reported in their study that skin tests remain of relevant importance in the work-up of a suspicion of hypersensitivity to BLA.
ConclusionA false label of BLA hypersensitivity will limit the use of more effective treatments and increase the consumption of more expensive antibiotics. Allergy work-up should be performed in patients with immediate reactions to BLA. Beta-lactam antibiotics hypersensitivity can be demonstrated in most patients via skin test. Thus, skin tests should be performed before a drug provocation test.