Crimean-Congo haemorrhagic fever (CCHF) is an acute, tick-borne viral disease. In temperate areas, CCHF cases occur between spring and early autumn when tick activity is high. This period is also the pollen season during which symptoms of allergic diseases are exacerbated. Viruses induce inflammatory and antiviral responses by binding to specific receptors on the surface of airway epithelial cells, resulting in activation of innate immune responses; release of mediators such as cytokines and chemokines; and recruitment of neutrophils and mononuclear cells to the area.
AimWe aimed to evaluate the frequency of self-reported allergic diseases and the effect on CCHF severity.
MethodBetween June and August 2008, a questionnaire was applied to 114 CCHF (+) patients and 122 healthy control subjects, 16 to 88 years old who attended the Infectious Diseases clinic and were hospitalised with CCHF suspected, by face to face interview including history of allergic rhinitis (AR), asthma symptoms and nonspecific bronchial reactivity, doctor diagnosed AR and/or asthma, and familial allergic diseases history.
ResultsAccording to PCR and/or enzyme-linked immunoassay (ELISA) results, 51.7% of patients (n=114) had CCHF. There was no significant relation between CCHF and history of AR, asthma symptoms and nonspecific bronchial reactivity, doctor diagnosed AR and/or asthma, and familial allergic diseases history. The severity of CCHF has not affected these parameters (p>.05). Of patients with positive CCHF test, 2.6% (n=3) and 3.5% (n=4) had doctor diagnosed AR and asthma, respectively.
ConclusionSelf-reported allergic diseases and CCHF are not related with each other.
Crimean-Congo haemorrhagic fever (CCHF) is a disease caused by a virus belonging to the Bunyaviridae family. CCHF virus isolation and/or disease have been reported from more than 30 countries in Africa, Asia, south-eastern Europe, and the Middle East. In 2001, a cluster of unidentified haemorrhagic fever cases was seen in a tertiary care hospital in Turkey. All the patients were from a small region of Middle Anatolia. Humans are infected mainly through direct contact with blood or tissues of viraemic hosts, or through tick bite or crushing infected ticks with unprotected hands.1–3
TNF-α and IL-6 are the cytokines most often detected during a CCHF viral infection. TNF-α was associated with the severe form of CCHF, while IL-6 was elevated in both severe and mild cases.4,5 There were significantly more peripheral blood natural killer cells in severe risk CCHF patients than in non-severe risk group CCHF patients.6
Asthma is a chronic heterogeneous inflammatory disease of the respiratory system in which numerous cytokines play a significant role. Among them TNF-α (tumour necrosis factor α), a proinflammatory cytokine, has a predominant role in orchestrating airway inflammation and affecting treatment outcome.7 The role of TNF-α in refractory asthma is strong. However, there was no effect of treatment with etanercept on airway responsiveness or lung function nor was there a clinically important effect on measures of eosinophilic and neutrophilic airway inflammation.8
Increased levels of IL-6 are documented in asthma, but their contribution to the pathology is unknown. Asthma is characterized by airway wall thickening due to increased extracellular matrix deposition, inflammation, angiogenesis, and airway smooth muscle (ASM) mass. IL-6 binds to a specific membrane-bound receptor. IL-6 trans-signalling in ASM has relevance to the development of airway remodelling.9
CCHF viruses are transmitted by Hyalomma genus ticks, particularly by Hyalomma marginatum marginatum. In the northern hemisphere, H. marginatum marginatum is usually activated by increasing temperature in the spring, particularly in April or May, and the immature stages are active in the summer between May and September.3 Aeroallergens are very often implicated in allergic rhinitis and asthma. Outdoor allergens (pollens) appear to constitute greater risk for seasonal rhinitis than indoor allergens. The grasses whose pollens cause the most common allergies, pollinate at the end of spring and the beginning of summer.10 This season is the activation period of CCHF viruses. The aim of this study is to evaluate the frequency of self-reported allergic diseases and the effect on CCHF severity with two diseases activated in the same season.
MethodsStudy populationThe study was carried out in the Cumhuriyet University, Faculty of Medicine, Chest Diseases Department and Allergic Diseases Subdepartment and Infection Diseases and Clinical Microbiology Department, Sivas, Turkey. A face-to-face interview questionnaire was administered to the consecutive 114 patients with CCHF who attended and were hospitalised in the Infection Diseases and Clinical Microbiology clinic during June and August 2008. All subjects agreed to answer this questionnaire.
The diagnosis of CCHF infection was based upon typical clinical and epidemiological findings and serological tests with ELISA and/or reverse transcriptase-polymerase chain reaction (RT-PCR). Serum samples of the patients were sent to the Virology Laboratory of Refik Saydam Hygiene Central Institute, Ankara, Turkey for microbiological testing for CCHF virus infection. Anti-CCHF IgM and IgG antibodies and CCHF viral antigen were tested in sera samples of patients by ELISA. The samples were also tested by RT-PCR.
Only patients with a definitive diagnosis of CCHF by means of clinical presentation plus the presence of specific IgM antibody by ELISA and/or detection of viral RNA by RT-PCR were included in the study.
The patients diagnosed CCHF evaluated for severity of diseases according to following criteria: White blood cell count of 10×10ψ cells per L or above, platelet count of 20×10ψ per L or below, aspartate aminotransferase level of 200U/L or over, alanine aminotransferase of 150U/L or over, activated partial thromboplastin time of 60s or more, or fibrinogen levels of 110mg/dl or under. The patients who satisfied one of these criteria were regarded as severe CCHF.11
Control groupA total of 122 control subjects who were recruited among persons who accompanied patients at the clinic answered the questionnaire. There were no significant differences in age, gender or smoking status between patients and control subjects.
QuestionnaireThe questionnaire survey sought demographic details, history of allergic rhinitis symptoms (Have you ever got rhinorrhoea, sneezing, nasal itching, nasal obstruction at April-May-June in previous years?), doctor-diagnosed allergic rhinitis (Have you ever got doctor-diagnosed allergic rhinitis (hay fever or spring rhinitis?), doctor-diagnosed asthma (Have you ever got doctor-diagnosed asthma?), history of asthma symptoms (Have you ever got asthma symptoms (wheezing, coughing, shortness of breath, chest tightness or pain) that wake you up at night or earlier than usual in the morning?), nonspecific bronchial hyperreactivity (“Have you ever got wheezing, cough or dyspnoea after exposure of cigarette smoke, bleacher, perfume or eau de cologne?”), dyspnoea after cold, familial allergic diseases history.
Statistical analysesStatistical analyses were performed using the Statistical Package for the Social Sciences version 10.0 for Windows (SPSS Inc, Chicago, IL, USA). Results of the study are presented as the mean±SEM. Univariate analysis is used for estimating differences and 95% confidence intervals (95% CI) between comparison groups is admitted. Categorical variables were analysed with the Pearson Chi-squared test or Fisher exact test. Simple differences between groups in continuous data were tested with Student's t test In all instances, a probability value less than 0.05 was considered as statistically significant.
Ethics approvalThe study was approved by the Cumhuriyet University, Faculty of Medicine Ethics Committee.
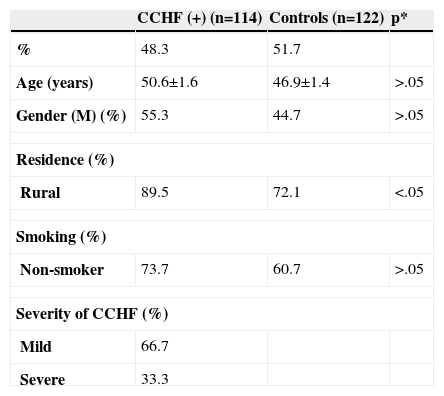
ResultsOf the 144 patients with suspected CCHF according to symptoms and hospitalised, 114 patients (79.1%) had CCHF. The mean age was 50.6±1.6 years (range 16.0–88.0yr) with 44.7% female of the patients with CCHF. The demographic characteristics of patients are shown in Table I.
Demographic characteristics of patients
| CCHF (+) (n=114) | Controls (n=122) | p* | |
| % | 48.3 | 51.7 | |
| Age (years) | 50.6±1.6 | 46.9±1.4 | >.05 |
| Gender (M) (%) | 55.3 | 44.7 | >.05 |
| Residence (%) | |||
| Rural | 89.5 | 72.1 | <.05 |
| Smoking (%) | |||
| Non-smoker | 73.7 | 60.7 | >.05 |
| Severity of CCHF (%) | |||
| Mild | 66.7 | ||
| Severe | 33.3 | ||
p<.05: Statistically significant.
Of the 114 patients with CCHF, 108 (94.7%) had positive RT-PCR and 99 (86.8%) had the presence of specific IgM antibody by ELISA. The mild and severe CCHF were observed in 76 (66.7%) and 38 (33.3%) of the subjects, respectively. The number of patients who lived in a rural area was higher in CCHF (+) patients than controls (p<0.05).
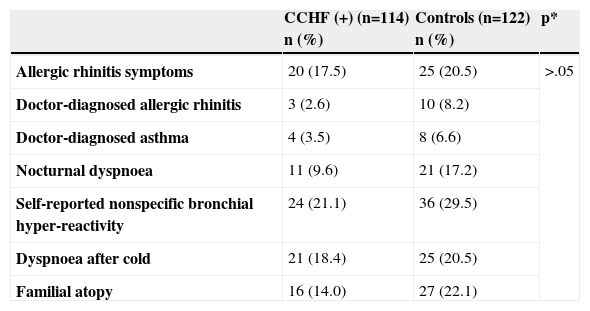
The frequency of self-reported allergic diseases is shown in Table II. There were no statistically significant differences in the self-reported allergic diseases between CCHF (+) patients and control subjects. The comparison of nonspecific bronchial hyper-reactivity (NSBHR) among female patients between CCHF (+) and control groups revealed that the percentage of presented self-reported NSBHR patients was lower in CCHF (+) group (21.6%, n=11 vs 45.5%, n=20; p<.013). There was no statistically significant difference in male patients between two groups.
Frequency of self reported allergic diseases
| CCHF (+) (n=114) n (%) | Controls (n=122) n (%) | p* | |
| Allergic rhinitis symptoms | 20 (17.5) | 25 (20.5) | >.05 |
| Doctor-diagnosed allergic rhinitis | 3 (2.6) | 10 (8.2) | |
| Doctor-diagnosed asthma | 4 (3.5) | 8 (6.6) | |
| Nocturnal dyspnoea | 11 (9.6) | 21 (17.2) | |
| Self-reported nonspecific bronchial hyper-reactivity | 24 (21.1) | 36 (29.5) | |
| Dyspnoea after cold | 21 (18.4) | 25 (20.5) | |
| Familial atopy | 16 (14.0) | 27 (22.1) |
p<.05: Statistically significant.
More male patients with severe CCHF had familial allergic diseases history than male patients with mild CCHF (18.2%, n=4 vs 2.4%, n=1; p=.046). A higher percentage of patients with history of dyspnoea after cold were male with severe CCHF compared with male with mild CCHF (31.8%, n=7; vs 9.8%, n=4; p=.039).
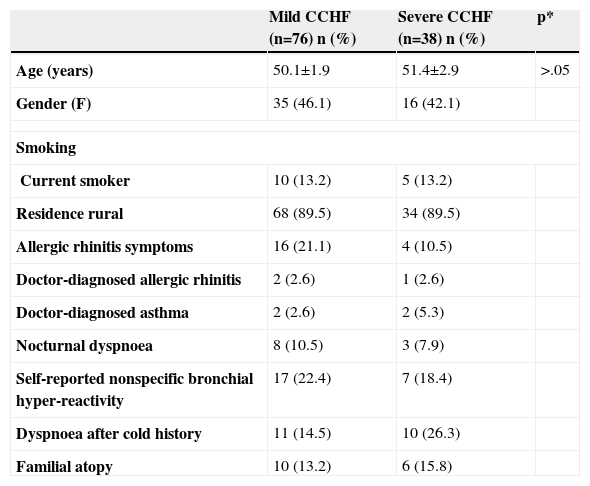
The frequency of self-reported allergic rhinitis symptoms, doctor-diagnosed AR, doctor-diagnosed asthma, history of asthma symptoms (dyspnoea, wheezing, cough), nonspecific bronchial reactivity, dyspnoea after colds, familial allergic diseases history were not statistically different between the patients with mild and severe CCHF (Table III).
The self-reported allergic diseases characteristics of patients with mild and severe CCHF
| Mild CCHF (n=76) n (%) | Severe CCHF (n=38) n (%) | p* | |
| Age (years) | 50.1±1.9 | 51.4±2.9 | >.05 |
| Gender (F) | 35 (46.1) | 16 (42.1) | |
| Smoking | |||
| Current smoker | 10 (13.2) | 5 (13.2) | |
| Residence rural | 68 (89.5) | 34 (89.5) | |
| Allergic rhinitis symptoms | 16 (21.1) | 4 (10.5) | |
| Doctor-diagnosed allergic rhinitis | 2 (2.6) | 1 (2.6) | |
| Doctor-diagnosed asthma | 2 (2.6) | 2 (5.3) | |
| Nocturnal dyspnoea | 8 (10.5) | 3 (7.9) | |
| Self-reported nonspecific bronchial hyper-reactivity | 17 (22.4) | 7 (18.4) | |
| Dyspnoea after cold history | 11 (14.5) | 10 (26.3) | |
| Familial atopy | 10 (13.2) | 6 (15.8) | |
This is the first report investigating the effect of CCHF to self-reported allergic diseases exacerbations and vice versa: the effect of self-reported allergic diseases history to CCHF severity.
Earlier studies mainly evaluated effects of respiratory syncytial virus on asthma.12 However, almost all of these studies designed in children patient groups. Rhinoviruses (RV) are a major cause of asthma exacerbations in children and adults. With the use of sensitive RT-PCR methods, respiratory viruses are found in approximately one half of such episodes in adults.13
We could not find any study in the literature which investigated the relationship between CCHF and allergic diseases. Thus, we were unable to compare our results with another study.
The percentage of persons who lived in a rural area was higher in CCHF group. However, the results of the present study show that there were no statistically significant difference in the self-reported allergic diseases between CCHF (+) patients and controls. It may be due to similar exposures and/or genetic features both of patients and controls.
The percentage of allergic rhinitis symptoms in patients with CCHF is consistent with the prevalence of allergic rhinitis in Turkey (17.5% in men and 21.2% in women vs 17.5% in CCHF (+) patients).14
In our study, there were no statistically significant difference in NSBHR and doctor diagnosed asthma between CCHF (+) and control groups. On the other hand, the percentage of dyspnoea after cold history in male patients with severe CCHF is found to be higher. The smoking status of the patients with severe CCHF was similar to that of male patients with mild CCHF. In contrast, there was lower NSBHR percentage in female patients with CCHF (+). This might be due to genetically and/or exposure differences between males and females. In addition, we could not perform methacholine bronchial provocation test to the patients in our study. In a previous study, Th1 responses to RV, as measured by IFN-γ or IFN-γ:IL-5 ratio, has been reported to correlate with methacholine PD20 and FEV1, respectively, but there was no relationship between RV-induced IL-5 and these measures of asthma severity. RV-induced IFN:IL-5 ratio and FEV1 are related with each other due to a deficient Th1 response rather than to an exuberant Th2 response, since minimal IL-5 is produced and it does not correlate with FEV1. In asthma, a defect in Th1 response is not limited to allergens but may also extend to viruses.15,16 Viral respiratory infections can cause increased airway responsiveness, although effects on the lower airway physiology are greatly influenced by characteristics of both the host and the virus. Viral infections could potentially cause bronchoconstriction and increased airway responsiveness by enhancing parasympathetic bronchoconstrictive responses, by stimulating reflex bronchospasm or neuropeptide release from sensory C fibres, or by interfering with the function of nonadrenergic, noncholinergic neurons, which produce the potent bronchodilator nitric oxide.17 Atopic asthmatic individuals seem to have a defective antiviral response which may be associated with incomplete viral clearance and persistent inflammation. In asthmatic children viral infections initiate bronchospasm and airway obstruction.17,18 However, in a prospective study it has been observed that although the duration of airway hyper-reactivity (AHR) after a single cold does not differ in atopic and non-atopic asthmatic children, an increased number of symptomatic colds cumulatively lead to prolonged AHR in the atopic group.19 In studies mentioned above include respiratory viruses. Data from these studies suggested that a single CCHF might not affect the existence of NSBHR.
There were some limitations in our study. Firstly, we could not perform skin prick test and methacholine bronchial provocation test. Since CCHF has high mortality rates and is highly contagious, it would not be ethical to perform tests unless the questionnaire results suggest a relation between CCHF and allergic diseases. Secondly, in order to prevent seasonal differences affecting the questionnaire results, our study is limited to a single season, which limits the number and types of patients as well.
In conclusion, our study demonstrates that self-reported allergic diseases and CCHF are not related with each other. Interestingly, there was a higher percentage of dyspnoea after cold history in male patients with severe CCHF and a lower percentage of presented self-reported NSBHR in female patients in CCHF (+) group. Further research in larger study populations is now required to confirm our findings.