Angiotensin Converting Enzyme inhibitors (ACEi) may cause angioedema, with an incidence of 0.1 % to 1 %, which may be life-threatening. ACEi induce angioedema by increasing the levels of bradykinin. Angiotensin II receptor blockers (ATRB), have a pharmacological profile similar to ACEi. The polymorphism of the ACE gene is based on the presence or absence of a 287-bp element on intron 16 on chromosome 17. The plasma level of ACE is related to gene polymorphism. ACE level in genotype DD is double that in genotype II.
ObjectiveThe aim of this study was to investigate whether the relationship between ACE gene polymorphism and ACEi induced angioedema is present or not.
MethodsACE gene polymorphism was investigated in patients with angioedema due to the use of ACEi or ATRB (n:32, group 1), in patients receiving ACEi or ATRB without angioedema (n:46, group 2), and healthy controls (n:96, group 3).
ResultsID polymorphism was the most frequent genotype in all groups, without any significant difference among the groups (p:0.868). ACE gene polymorphism was not related with the drugs used (ACEi or ATRB), localisation of angioedema, and female sex, in group 1.
ConclusionOur results showed that ACE gene polymorphism has no effect on ACEi or ATRB induced angioedema.
Angioedema is described as a swelling that involves skin or mucosal membrane, or respiratory and gastrointestinal system epithelium.1,2 Angioedema frequently occurs with urticaria, but can also happen as an isolated finding itself. Angioedema due to ACEi and ATRB mostly presents as an isolated finding without urticaria.
ACE is a two membrane-bound zinc-containing metallopeptidase and it has widespread tissue distribution including the vascular endothelium and smooth muscle cells, cardiac myocytes and fibroblasts, the kidney and the brain.3
ACEi exert their effects on the regulation of blood pressure and electrolyte balance as it converts Angiotensin I (AT I) to AT II. AT II is a potent vasoconstrictor, and increases blood pressure. ACEi are used frequently in the treatment of hypertension, congestive heart failure, and diabetic nephropathy.4–6 Cardiac effects of ACEi are the prevention of cardiac hypertrophy and the reduction in infarct size.3 ACE inhibition also increases kinin peptide levels. Kinins are potent vasodilators, promote diuresis and natriuresis, and have cardioprotective actions. However, high levels of kinin peptides produce inflammation and uncommonly ACEi produce marked elevation of kinin peptide levels, resulting in angioedema.3 The rate of angioedema in patients receiving ACEi was found to range between 0.1-0.7%.1,2,7–12 Although the patient declared rate is low, it is possible to observe angioedema in more patients than expected considering the widespread use of these drugs. Although the exact mechanism of ACEi induced angioedema is not known, bradykinin and substance P have been implicated in the pathogenesis of angioedema.13,14 ACEi precipitate attacks by directly interfering with the degradation of bradykinin, thereby potentiating its biological effect. Bradykinin is a nanopeptide which shows a strong vasoactive activity on human skin.15 Bradykinin concentration is increased in ACEi related angioedema due to reduced bradykinin catabolism rather than to increased bradykinin production.14,16,17 Nevertheless ATRB which do not affect bradykinin levels may also cause angioedema. There is still no marker to predict which patients using ACEi will develop angioedema. Carboxypeptidase N (CPN) is responsible for the transformation of bradykinin into its active metabolite des-arginine-bradykinin. This metabolite has a poor affinity for B2 receptors. Des-arg bradykinin level is increased in ACE induced angioedema.18 It was suggested that a decrease in degradation of des-arg-bradykinin might play a role in ACEi dependent angioedema in patients with hypertension.19 The plasma activity and levels of enzymes such as CPN and aminopeptidase P (APP) were found to be low in patients with angioedema.15,17,19 In a genetic study, C-2399A variant in XPNPEP2 -which is candidate gene encoding membrane-bound APP- is associated with reduced APP activity and higher incidence of ACEi induced angioedema.17 Dipeptidyl peptidase IV (DPPIV) activity is decreased in patients with ACEi induced angioedema.13
ACE gene is localized in the 17th chromosome in humans. Polymorphism is detected in an area with 286 bases in the 16th intron of ACE gene.6,19–25 The average serum ACE level on patients with deletion/deletion (DD) genotype is more than on patients with insertion/insertion (II) genotype. ACE gene polymorphism was found to be correlated with hypertension,26 physical performance,27 left ventricle hypertrophy,28 and diabetic nephropathy29 in different societies. Up to our current knowledge, the association between angioedema due to ACE inhibitor and ACE gene polymorphism has never been studied. The aim of this study was to investigate whether the relationship between ACE gene polymorphism and ACEi induced angioedema is present or not.
METHODSThis study was conducted as a single-centre study in the Department of Allergy of Gulhane Military Medical Academy between 2003 and 2006. Patients informed consent and local ethic committee approval was received.
Patient SelectionPatients admitted to hospital with angioedema were evaluated and among them ACEI and/or ATRB drug users were registered to this study. Medical history (using drugs, onset of reaction time, drug period, and association of angioedema with taking drug) were analysed and thorough physical examinations were performed. The mucosal regions in which reaction was observed were determined. We excluded patients using non-steroidal anti-inflammatory drugs and acetylsalicylic acid. Also, hereditary angioedema inquiry was made. When patients interrogated for using diuretic combination, single use of diuretic was not associated with angioedema, however, after adding ACE or ARB, reactions became visible. On the basis of this finding, patients were enrolled to the study group. Patients were also asked for sulphonamides allergy. Furthermore, diuretic associated angioedema has not been detected without cross reaction to sulphonamides up to our knowledge. The patients who were included in Group 1 had been followed for essential hypertension. Patients with secondary hypertension were excluded from the study.
Laboratory testsComplete blood count, routine biochemical tests, erythrocyte sedimentation rate, TSH, Anti-TPO, C3, C4, HBsAg, Anti-HCV were performed on all patients with angioedema. Also, mixed epidermal prick test (grass mix, cereals, tree mix-I, tree mix-II, cockroach, mould mix, weed mix, latex, Der. Farinea, Der. Pteronyssinus, dog epitelia, cat epitelia. Allergopharma D-21462 Reinbek) and food prick test (40 food antigens Allergopharma D-21462 Reinbek) were performed.
After all these evaluations, patients who had no other risk factors to cause angioedema were included in the study group. Patients who experienced angioedema during ACEi or ATRB user were selected as the patient group (Group 1, n: 32).
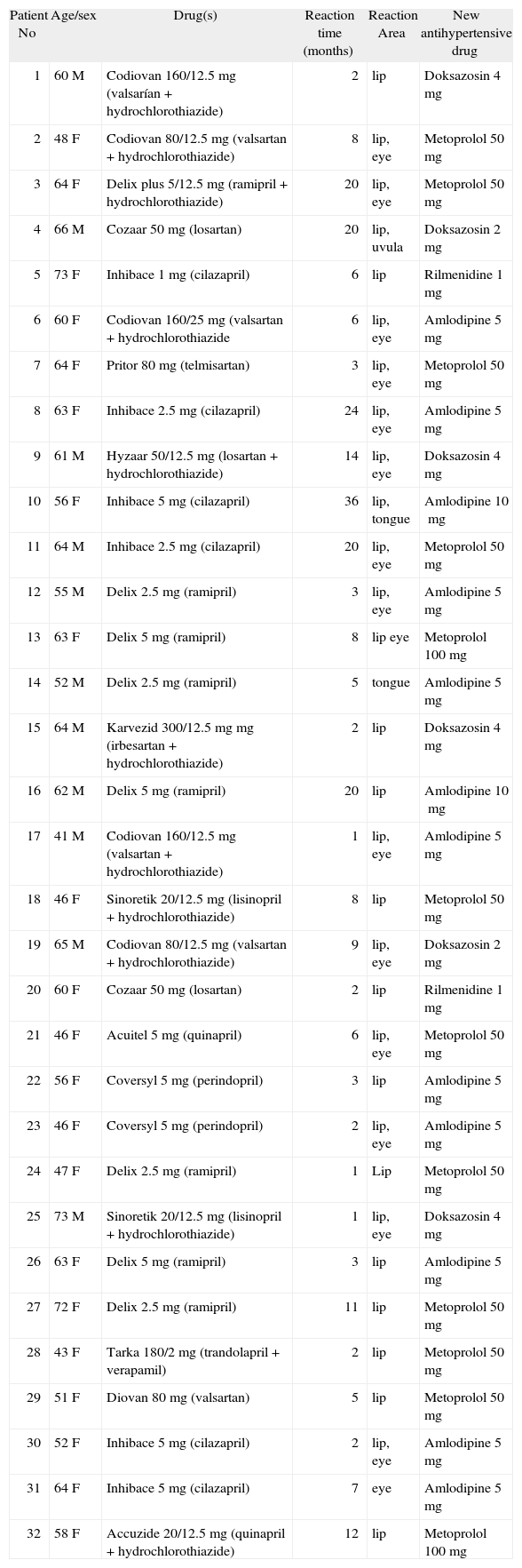
ACEi and ATRB drugs which are responsible for angioedema were substituted with alternative antihypertensive drugs. The drugs which were administrated after changing the treatment are shown in table I. Due to the risk of laryngeal edema, challenge test was not applied.
Characteristics of patients in Group 1
| Patient No | Age/sex | Drug(s) | Reaction time (months) | Reaction Area | New antihypertensive drug |
| 1 | 60M | Codiovan 160/12.5mg (valsarían + hydrochlorothiazide) | 2 | lip | Doksazosin 4mg |
| 2 | 48F | Codiovan 80/12.5mg (valsartan + hydrochlorothiazide) | 8 | lip, eye | Metoprolol 50mg |
| 3 | 64F | Delix plus 5/12.5mg (ramipril + hydrochlorothiazide) | 20 | lip, eye | Metoprolol 50mg |
| 4 | 66M | Cozaar 50mg (losartan) | 20 | lip, uvula | Doksazosin 2mg |
| 5 | 73F | Inhibace 1mg (cilazapril) | 6 | lip | Rilmenidine 1mg |
| 6 | 60F | Codiovan 160/25mg (valsartan + hydrochlorothiazide | 6 | lip, eye | Amlodipine 5mg |
| 7 | 64F | Pritor 80mg (telmisartan) | 3 | lip, eye | Metoprolol 50mg |
| 8 | 63F | Inhibace 2.5mg (cilazapril) | 24 | lip, eye | Amlodipine 5mg |
| 9 | 61M | Hyzaar 50/12.5mg (losartan + hydrochlorothiazide) | 14 | lip, eye | Doksazosin 4mg |
| 10 | 56F | Inhibace 5mg (cilazapril) | 36 | lip, tongue | Amlodipine 10mg |
| 11 | 64M | Inhibace 2.5mg (cilazapril) | 20 | lip, eye | Metoprolol 50mg |
| 12 | 55M | Delix 2.5mg (ramipril) | 3 | lip, eye | Amlodipine 5mg |
| 13 | 63F | Delix 5mg (ramipril) | 8 | lip eye | Metoprolol 100mg |
| 14 | 52M | Delix 2.5mg (ramipril) | 5 | tongue | Amlodipine 5mg |
| 15 | 64M | Karvezid 300/12.5mgmg (irbesartan + hydrochlorothiazide) | 2 | lip | Doksazosin 4mg |
| 16 | 62M | Delix 5mg (ramipril) | 20 | lip | Amlodipine 10mg |
| 17 | 41M | Codiovan 160/12.5mg (valsartan + hydrochlorothiazide) | 1 | lip, eye | Amlodipine 5mg |
| 18 | 46F | Sinoretik 20/12.5mg (lisinopril + hydrochlorothiazide) | 8 | lip | Metoprolol 50mg |
| 19 | 65M | Codiovan 80/12.5mg (valsartan + hydrochlorothiazide) | 9 | lip, eye | Doksazosin 2mg |
| 20 | 60F | Cozaar 50mg (losartan) | 2 | lip | Rilmenidine 1mg |
| 21 | 46F | Acuitel 5mg (quinapril) | 6 | lip, eye | Metoprolol 50mg |
| 22 | 56F | Coversyl 5mg (perindopril) | 3 | lip | Amlodipine 5mg |
| 23 | 46F | Coversyl 5mg (perindopril) | 2 | lip, eye | Amlodipine 5mg |
| 24 | 47F | Delix 2.5mg (ramipril) | 1 | Lip | Metoprolol 50mg |
| 25 | 73M | Sinoretik 20/12.5mg (lisinopril + hydrochlorothiazide) | 1 | lip, eye | Doksazosin 4mg |
| 26 | 63F | Delix 5mg (ramipril) | 3 | lip | Amlodipine 5mg |
| 27 | 72F | Delix 2.5mg (ramipril) | 11 | lip | Metoprolol 50mg |
| 28 | 43F | Tarka 180/2mg (trandolapril + verapamil) | 2 | lip | Metoprolol 50mg |
| 29 | 51F | Diovan 80mg (valsartan) | 5 | lip | Metoprolol 50mg |
| 30 | 52F | Inhibace 5mg (cilazapril) | 2 | lip, eye | Amlodipine 5mg |
| 31 | 64F | Inhibace 5mg (cilazapril) | 7 | eye | Amlodipine 5mg |
| 32 | 58F | Accuzide 20/12.5mg (quinapril + hydrochlorothiazide) | 12 | lip | Metoprolol 100mg |
Control group consisted of the patients without angioedema taking neither ACEi nor ATRB. Patients without angioedema under treatment with ACEi or ATRB constituted the control group (Group 2, n: 46). The drugs which were used in Group 2 are shown in table II. In addition, volunteers who did not have any history of chronic diseases and did not use the previously mentioned drugs were enrolled in this study. Healthy volunteers formed the control group (Group 3, n: 96).
Drugs used in Group 2 (without angioedema)
| Patient No | Age/sex | Drug(s) |
| 1 | 59F | Accuzide 20/12.5mg (quinapril + hydrochlorothiazide) |
| 2 | 66M | Delix 5mg (ramipril) |
| 3 | 48M | Inhibace 5mg (cilazapril) |
| 4 | 59M | Inhibace 5mg (cilazapril) |
| 5 | 53F | Delix 2.5mg (ramipril) |
| 6 | 75M | Coversyl 5mg (perindopril) |
| 7 | 80F | Delix 5mg (ramipril) |
| 8 | 57F | Inhibace 2.5mg (cilazapril) |
| 9 | 72M | Karvezide 300/12.5mgmg (irbesartan + hydrochlorothiazide) |
| 10 | 65F | Diovan 80mg (valsartan) |
| 11 | 61F | Hyzaar 50/12.5mg (losartan + hydrochlorothiazide) |
| 12 | 61F | Eklips 50/12.5mg (losartan + hydrochlorothiazide) |
| 13 | 69F | Acuitel 5mg (quinapril) |
| 14 | 58M | Delix 2.5mg (ramipril) |
| 15 | 65M | Zestoretic 20/12.5mg (lisinopril + hydrochlorothiazide) |
| 16 | 78M | Hyzaar 50/12.5mg (losartan + hydrochlorothiazide) |
| 17 | 39M | Delix 2.5mg (ramipril) |
| 18 | 59F | Vasolapril 10mg (enalapril) |
| 19 | 74M | Diovan 80mg (valsartan) |
| 20 | 51F | Diovan 160mg (valsartan) |
| 21 | 32F | Diovan 80mg (valsartan) |
| 22 | 47F | Diovan 80mg (valsartan) |
| 23 | 50F | Coversyl 5mg (perindopril) |
| 24 | 77M | Rilace 20mg (lisinopril) |
| 25 | 58F | Diovan 80mg (valsartan) |
| 26 | 41F | Micardis 80mg (telmisartan) |
| 27 | 73M | Inhibace 5mg (cilazapril) |
| 28 | 42M | Coversyl 5mg (perindopril) |
| 29 | 54F | Delix 2.5mg (ramipril) |
| 30 | 65F | Cozaar 50mg (losartan) |
| 31 | 70F | Eklips 50/12.5mg (losartan + hydrochlorothiazide) |
| 32 | 53F | Rilace 20mg (lisinopril) |
| 33 | 52F | Hyzaar 50/12.5mg (losartan + hydrochlorothiazide) |
| 34 | 47F | Diovan 80mg (valsartan) |
| 35 | 66F | Delix 5mg (ramipril) |
| 36 | 68M | Cozaar 50mg (losartan) |
| 37 | 63F | Cozaar 50mg (losartan) |
| 38 | 77F | Inhibace 5mg (cilazapril) |
| 39 | 77F | Delix 5mg (ramipril) |
| 40 | 63F | Diovan 160mg (valsartan) |
| 41 | 53F | Sinoretic 20mg (lisinopril) |
| 42 | 51F | Diovan 80mg (valsartan) |
| 43 | 60F | Inhibace 2.5mg (cilazapril) |
| 44 | 64F | Pritor 80mg (telmisartan) |
| 45 | 58F | Ayra 8mg (candesartan) |
| 46 | 55F | Gopten 2mg (trandolapril) |
We collected 5cc of blood in EDTA tube from patient group and each of the two control groups. Blood samples were transported to the genetic laboratory on the same day, the blood samples were saved at −20°C after DNA isolation. The blood samples were studied by the same person in the same working session.
DNA isolationThe 16th intron of the ACE gene from peripheral blood was multiplied using Genomic DNA isolation kit (Genemark, No: DP023-50, Hopegen Biotechnology Development Enterprise-Taiwan), and was examined and scanned with gel electrophoresis under UV. To multiply the studied gene area, sense; 5′-CTG CAG ACC ACT CCC ATC CTT TCT-3′ and anti-sense; 5′-GAT GTG GCC ATC ACA TTC GTC AGA-3′ primers were used. The bands received on gel were detected inversion being 335bc, deletion being 190bc.
Statistical AnalysisStatistical evaluations were performed by using SPSS for Windows version 11.0 (Chi, III, USA) software package. Results were reported as the mean value ± sd rate and as percentage. One-way ANOVA analysis and chi square test were applied for evaluation of the importance of differences among the groups. A p value < 0.05 was considered as statistically significant.
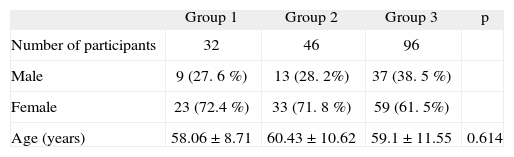
RESULTSOne hundred and seventy-four participants (115 female and 59 male) were enrolled in the study. The mean age was 59.21 ± 10.81years (39 to 92). The distributions of gender and ages between the groups were similar (table III).
Distribution of sex and age among groups
| Group 1 | Group 2 | Group 3 | p | |
| Number of participants | 32 | 46 | 96 | |
| Male | 9 (27. 6 %) | 13 (28. 2%) | 37 (38. 5 %) | |
| Female | 23 (72.4 %) | 33 (71. 8 %) | 59 (61. 5%) | |
| Age (years) | 58.06 ± 8.71 | 60.43 ± 10.62 | 59.1 ± 11.55 | 0.614 |
Group 1: Patients with angioedema during ACEi or ATRB therapy.
Group 2: Patients without angioedema during ACEi or ATRB therapy.
Group 3: Healthy control group.
p (One way ANOVA analysis).
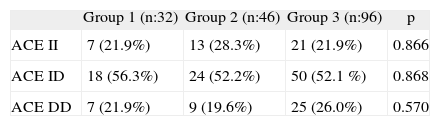
With respect to the ACE genotype there was no significant difference between the groups (table IV).
The distribution of ACE genotype among groups
| Group 1 (n:32) | Group 2 (n:46) | Group 3 (n:96) | p | |
| ACE II | 7 (21.9%) | 13 (28.3%) | 21 (21.9%) | 0.866 |
| ACE ID | 18 (56.3%) | 24 (52.2%) | 50 (52.1 %) | 0.868 |
| ACE DD | 7 (21.9%) | 9 (19.6%) | 25 (26.0%) | 0.570 |
II: Insertion/ insertion polymorphism.
DD: Deletion/ deletion polymorphism.
ID: Insertion/ deletion polymorphism.
p (chi square test).
The drugs which caused angioedema, the onset of symptoms and angioedema localization, the alternative drugs given and the age and gender of patients is summarised in table I. There is a slight dominance of ID genotype in all groups, about half of patients in Group 1 (n: 18). The time of onset of angioedema after the drug(s) introduction was less than 6months. The earliest reaction was on a patient on the first day, while the latest one was after 36months.
The distribution of the affected body parts with respect to ACE genotypes was also evaluated. The most frequently affected site was lips; however, there was no significance between ACE genotypes.
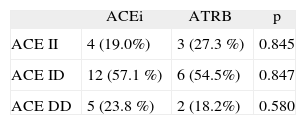
In group 1, 21 patients (65 %) were used ACEi and 11 patients (35 %) ATRB, while this ratio was 25 (55 %) and 21 (45 %) in group 2 respectively.
The patients with angioedema were compared with respect to used drugs and no significance was observed (table V).
DISCUSSIONIn this study, the relation between angioedema due to ACEi and ATRB and ACE gene polymorphism was investigated. To our knowledge there has been no previous study addressing this issue. However, possible relation between the ACE genotype and angioedema has previously been mentioned in one study.8
ACE gene polymorphism has been studied in many diseases, even in physiological conditions, and connections have been looked for between genotype and clinic.
ACE gene polymorphism had been evaluated in asthma, and DD genotype in patients who suffered from asthma was found to be significantly higher, compared with the control group.30 In another study on asthma, high DD genotype was demonstrated not to have a meaningful effect. 21
Behcet disease is basically a vasculitis in which endothelial dysfunction is evident. Because the tissue renin angiotensin system is effective on endothelium, ACE gene polymorphism has been studied on these patients, and no genotype has been found to be dominant.22
Patients who suffered ACEi induced angioedema were older than the other angioedema groups.2 Nearly half of the cases appear in the first week of the treatment, but lesion can appear later as well.2,31 In our study in 18 (56.4 %) of the patients, reactions were observed in the first 6month period.
It was demonstrated in retrospective studies that angioedema was observed more on blacks.2,32 The increased risk in black patients may be related to racial differences in the kallikrein-kinin system and increased sensitivity to bradykinin.17,32
When ACEi were evaluated one by one, angioedema was found to be associated with the group but not to the drug itself.2 The provocation test with ACEi or ATRB to confirm the diagnosis was not applied due to ethical considerations. In addition, a patient had used ACEi mistakably and tongue and uvula edema repeated in a short period, the patient was kept under watch at a hospital for 24 hours, in case the clinical situation were severe.
Angioedema related to ACEi usually appears above the neck, around the face area, but the reason for this is not known. Angioedema was observed in the head and neck region in all patients in our study. Serious complications can happen related to tongue and oropharyngeal and sometimes speech defect may occur.1,2,9 Reactions can be fatal, and could need intubation.33 In none of our cases intubation is indicated. Angioedema on the lip was observed mostly. For instance, extraordinary situations can cause wrong diagnosis and treatment. One patient attended the emergency room for shortness of breath, but was diagnosed as a case of panic attack. Tongue edema in a patient with thyroidectomy considered as a myxedema. The same patient was examined for speech impairment and hoarseness. All these symptoms and findings ceased when ACEi stopped and angioedema treatment was applied briefly.
When angioedema related with the use of ACEi was first noted, it was thought that the best alternative treatment was ATRB. But this treatment caused the reappearance of angioedema and also the ATRB were not safe. In literature, there are many reports about this.34–37 Also in our study, in 11 patients ATRB related angioedema were reported during the three year follow-up period. After ACEi treatment had been stopped, ATRB were not started to these patients.
It is known that the ATRB exert their antihypertensive effects by selectively binding to their angiotensin receptors.35,38 In contrast to ACEi, it is supposed that the ATRB do not affect bradykinin levels. However, the mechanism of developing angioedema with ATRB has not been still identified.35 Probable mechanisms are rising bradykinin or metabolites in the circulation, and the effect of kininase-I enzyme which plays a role in hereditary angioedema pathophysiology may reduce as a result of ATRB treatment.35
It was determined that angioedema dependent on ACEi and ATRB was higher in blacks,39 and II genotype was dominant in blacks as well. All the patients included in this study are Caucasian and ID is dominant in genotype distribution. In a study carried out with healthy Turkish individuals, it had been observed that ID genotype was dominant.22
Angioedema due to ACEi and ATRB are not usually taken under control following cease of the drug with antihistamines and corticosteroid treatment,17 although some patients may respond to treatment. As a matter of fact this condition was stated in literature. In one article, a patient of African origin with angioedema who did not respond to corticosteroid, antihistamine, and adrenaline had been described. This patient had been treated with fresh-frozen plasma infusion.7 Fresh-frozen plasma supplies ACE to patient and accelerates the breakdown of bradykinin.
The incidence of a disease or a side effect of a drug in a population is not the only factor determining its importance; also the morbidity and mortality rate are as important as its incidence. Reducing probable side effects on patients is an issue that can be appraised by preventive medicine. In daily practice, doctors pay close attention to known contraindications and relative contraindications, as they prescribe.
Angioedema due to ACEi and ATRB, when evaluated statistically, is a rare complication. But sometimes it could be as serious as a fatal complication that can lead to loss of life of patients. If the relation between ACE gene polymorphism or single nucleotide mutations determined by further research and development of angioedema can be found, patients can be treated with selective drug treatments. Even though pharmacogenomic studies focus especially on chemotherapy resistance in cancer treatment, ACE activity difference related to genotype and widespread using of drugs effecting the above enzyme are going to be an important study field in the future. In today's treatment planning, specific drugs are suggested to patient groups with a similar diagnosis. Side effects and overall effects of drugs show different results on the patients. In our opinion, ACEi related angioedema is going to be a new field of study for pharmacogenomic studies which research the place of genetics in individual drug treatments.