Recently, a great deal of attention has been paid to the investigation of regulatory functions of microRNA. Currently, many different mechanisms involved in the pathogenesis of asthma are known, but the whole picture of pathogenesis has not yet been studied.
ConclusionsMicroRNAs play an important role in the regulation of many cellular processes. Undoubtedly, these regulatory molecules are involved in the pathogenesis of asthma, and therefore can be potential targets for treatment.
Asthma is a chronic inflammatory airway disease that occurs as a reaction to inhaled antigens, such as respiratory viruses, allergens or air pollutants, which leads to airway inflammation, excessive airway hypersensitiveness and reversible airflow obstruction. The key link in bronchial asthma is bronchial obstruction.2
The pathogenesis of bronchial asthma is based on a non-specific increase in the bronchial hyper-reactivity. The higher the bronchial hyper-reactivity the more serious the disease is and the more difficult the treatment is.9,21 It is still unknown why the bronchial hyper-reactivity increases in patients with bronchial asthma. An important role in the pathogenesis is played by mast cells, eosinophils, macrophages, neutrophils and lymphocytes. Mediators of inflammation that are released during degranulation of mast cells – histamine, bradykinin, leukotrienes C, D and E, etc. – cause a spasm of smooth muscles of the bronchi, vasodilation and mucosal edema. In addition, leukotrienes cause mucus secretion and mucociliary transport disorders, which creates conditions for the transition of acute inflammation to chronic inflammation.7 Also, T-lymphocytes play an important role in the development of bronchial asthma.1 There are many T-lymphocytes in bronchi in patients with bronchial asthma. They secrete cytokines and participate in the regulation of cellular and humoral immunity. Th1 produces IL-2 and IFNγ, which stimulate the proliferation and differentiation of T-lymphocytes and activating macrophages. Th2 produce IL-4, -5, -10, -13, which stimulate the proliferation of B-lymphocytes and synthesis of immunoglobulins. In addition, IL-5 stimulates the proliferation, differentiation and activation of eosinophils, and possibly the degranulation of basophils.1,20 Currently, many different mechanisms involved in the pathogenesis of asthma are known, but the whole picture of pathogenesis has not yet been studied. One of the key molecules that can participate in the regulation of these processes is microRNA, which are important regulatory molecules of intracellular processes. The cells of the immune system are also regulated by microRNA and other regulatory structures.
The immune system is controlled by a coordinated system of regulation of gene expression in each type of involved cells. One of the regulators of gene expression are microRNAs (miRNAs).6,18 Every year more and more studies are conducted on the subject of the functions of these molecules.2 These studies show that miRNAs control the signaling pathways in each cell type, affect the development and phenotypic stability of immune cells, and regulate the intensity of inflammation in the tissues. The biological functions of miRNA are numerous and diverse, so the regulation of the expression of these molecules can potentially be used for therapeutic purposes. Many studies show that miRNAs can potentially be used as non-invasive biomarkers to better recognize types of heterogeneous diseases, including asthma and other allergic diseases.2,6 In recent years, there have been many studies confirming the involvement of miRNAs in the development of allergic diseases. In this article, we present an overview of miRNAs that play a role in the development of bronchial asthma (Table 1).
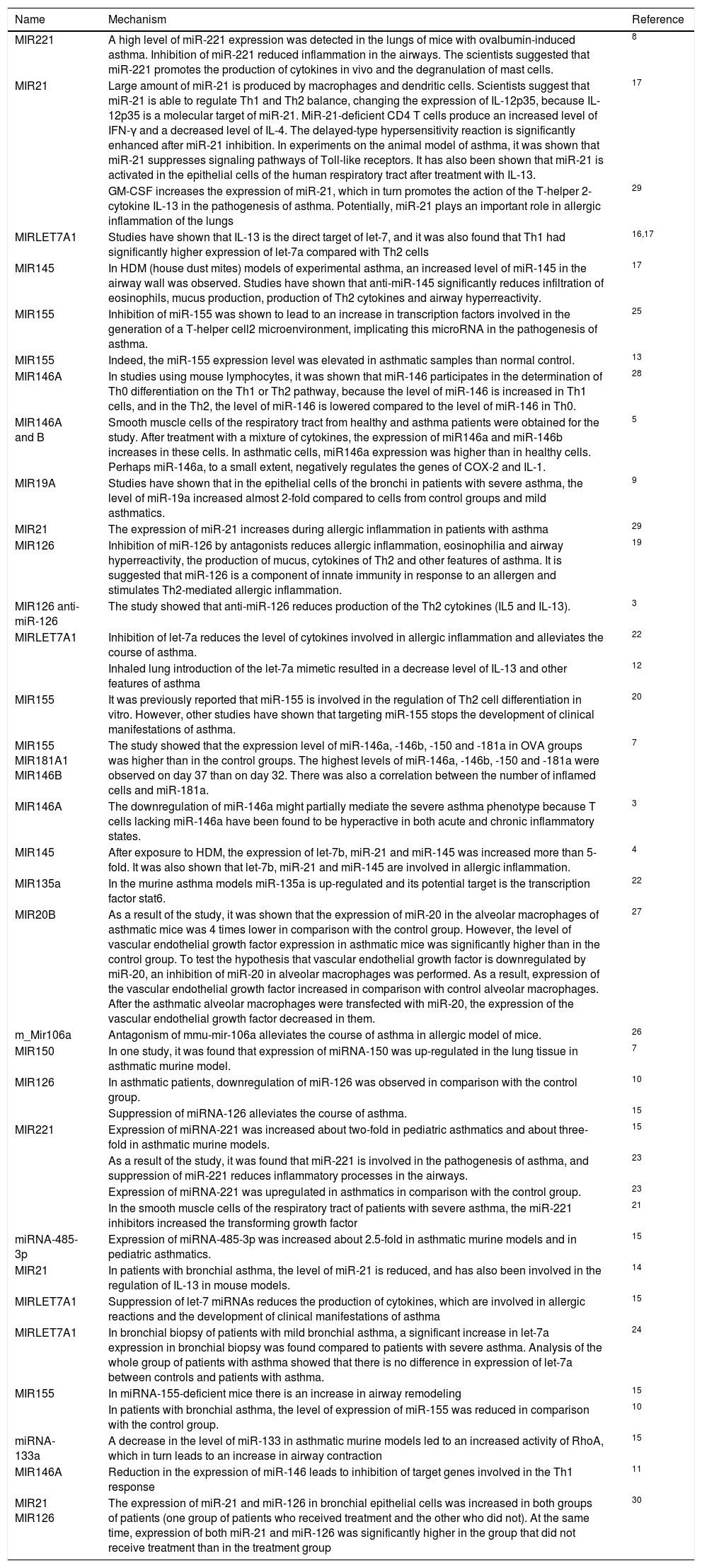
The role of miRNAs in the pathogenesis of asthma.
| Name | Mechanism | Reference |
|---|---|---|
| MIR221 | A high level of miR-221 expression was detected in the lungs of mice with ovalbumin-induced asthma. Inhibition of miR-221 reduced inflammation in the airways. The scientists suggested that miR-221 promotes the production of cytokines in vivo and the degranulation of mast cells. | 8 |
| MIR21 | Large amount of miR-21 is produced by macrophages and dendritic cells. Scientists suggest that miR-21 is able to regulate Th1 and Th2 balance, changing the expression of IL-12p35, because IL-12p35 is a molecular target of miR-21. MiR-21-deficient CD4 T cells produce an increased level of IFN-γ and a decreased level of IL-4. The delayed-type hypersensitivity reaction is significantly enhanced after miR-21 inhibition. In experiments on the animal model of asthma, it was shown that miR-21 suppresses signaling pathways of Toll-like receptors. It has also been shown that miR-21 is activated in the epithelial cells of the human respiratory tract after treatment with IL-13. | 17 |
| GM-CSF increases the expression of miR-21, which in turn promotes the action of the T-helper 2-cytokine IL-13 in the pathogenesis of asthma. Potentially, miR-21 plays an important role in allergic inflammation of the lungs | 29 | |
| MIRLET7A1 | Studies have shown that IL-13 is the direct target of let-7, and it was also found that Th1 had significantly higher expression of let-7a compared with Th2 cells | 16,17 |
| MIR145 | In HDM (house dust mites) models of experimental asthma, an increased level of miR-145 in the airway wall was observed. Studies have shown that anti-miR-145 significantly reduces infiltration of eosinophils, mucus production, production of Th2 cytokines and airway hyperreactivity. | 17 |
| MIR155 | Inhibition of miR-155 was shown to lead to an increase in transcription factors involved in the generation of a T-helper cell2 microenvironment, implicating this microRNA in the pathogenesis of asthma. | 25 |
| MIR155 | Indeed, the miR-155 expression level was elevated in asthmatic samples than normal control. | 13 |
| MIR146A | In studies using mouse lymphocytes, it was shown that miR-146 participates in the determination of Th0 differentiation on the Th1 or Th2 pathway, because the level of miR-146 is increased in Th1 cells, and in the Th2, the level of miR-146 is lowered compared to the level of miR-146 in Th0. | 28 |
| MIR146A and B | Smooth muscle cells of the respiratory tract from healthy and asthma patients were obtained for the study. After treatment with a mixture of cytokines, the expression of miR146a and miR-146b increases in these cells. In asthmatic cells, miR146a expression was higher than in healthy cells. Perhaps miR-146a, to a small extent, negatively regulates the genes of COX-2 and IL-1. | 5 |
| MIR19A | Studies have shown that in the epithelial cells of the bronchi in patients with severe asthma, the level of miR-19a increased almost 2-fold compared to cells from control groups and mild asthmatics. | 9 |
| MIR21 | The expression of miR-21 increases during allergic inflammation in patients with asthma | 29 |
| MIR126 | Inhibition of miR-126 by antagonists reduces allergic inflammation, eosinophilia and airway hyperreactivity, the production of mucus, cytokines of Th2 and other features of asthma. It is suggested that miR-126 is a component of innate immunity in response to an allergen and stimulates Th2-mediated allergic inflammation. | 19 |
| MIR126 anti-miR-126 | The study showed that anti-miR-126 reduces production of the Th2 cytokines (IL5 and IL-13). | 3 |
| MIRLET7A1 | Inhibition of let-7a reduces the level of cytokines involved in allergic inflammation and alleviates the course of asthma. | 22 |
| Inhaled lung introduction of the let-7a mimetic resulted in a decrease level of IL-13 and other features of asthma | 12 | |
| MIR155 | It was previously reported that miR-155 is involved in the regulation of Th2 cell differentiation in vitro. However, other studies have shown that targeting miR-155 stops the development of clinical manifestations of asthma. | 20 |
| MIR155 MIR181A1 MIR146B | The study showed that the expression level of miR-146a, -146b, -150 and -181a in OVA groups was higher than in the control groups. The highest levels of miR-146a, -146b, -150 and -181a were observed on day 37 than on day 32. There was also a correlation between the number of inflamed cells and miR-181a. | 7 |
| MIR146A | The downregulation of miR-146a might partially mediate the severe asthma phenotype because T cells lacking miR-146a have been found to be hyperactive in both acute and chronic inflammatory states. | 3 |
| MIR145 | After exposure to HDM, the expression of let-7b, miR-21 and miR-145 was increased more than 5-fold. It was also shown that let-7b, miR-21 and miR-145 are involved in allergic inflammation. | 4 |
| MIR135a | In the murine asthma models miR-135a is up-regulated and its potential target is the transcription factor stat6. | 22 |
| MIR20B | As a result of the study, it was shown that the expression of miR-20 in the alveolar macrophages of asthmatic mice was 4 times lower in comparison with the control group. However, the level of vascular endothelial growth factor expression in asthmatic mice was significantly higher than in the control group. To test the hypothesis that vascular endothelial growth factor is downregulated by miR-20, an inhibition of miR-20 in alveolar macrophages was performed. As a result, expression of the vascular endothelial growth factor increased in comparison with control alveolar macrophages. After the asthmatic alveolar macrophages were transfected with miR-20, the expression of the vascular endothelial growth factor decreased in them. | 27 |
| m_Mir106a | Antagonism of mmu-mir-106a alleviates the course of asthma in allergic model of mice. | 26 |
| MIR150 | In one study, it was found that expression of miRNA-150 was up-regulated in the lung tissue in asthmatic murine model. | 7 |
| MIR126 | In asthmatic patients, downregulation of miR-126 was observed in comparison with the control group. | 10 |
| Suppression of miRNA-126 alleviates the course of asthma. | 15 | |
| MIR221 | Expression of miRNA-221 was increased about two-fold in pediatric asthmatics and about three-fold in asthmatic murine models. | 15 |
| As a result of the study, it was found that miR-221 is involved in the pathogenesis of asthma, and suppression of miR-221 reduces inflammatory processes in the airways. | 23 | |
| Expression of miRNA-221 was upregulated in asthmatics in comparison with the control group. | 23 | |
| In the smooth muscle cells of the respiratory tract of patients with severe asthma, the miR-221 inhibitors increased the transforming growth factor | 21 | |
| miRNA-485-3p | Expression of miRNA-485-3p was increased about 2.5-fold in asthmatic murine models and in pediatric asthmatics. | 15 |
| MIR21 | In patients with bronchial asthma, the level of miR-21 is reduced, and has also been involved in the regulation of IL-13 in mouse models. | 14 |
| MIRLET7A1 | Suppression of let-7 miRNAs reduces the production of cytokines, which are involved in allergic reactions and the development of clinical manifestations of asthma | 15 |
| MIRLET7A1 | In bronchial biopsy of patients with mild bronchial asthma, a significant increase in let-7a expression in bronchial biopsy was found compared to patients with severe asthma. Analysis of the whole group of patients with asthma showed that there is no difference in expression of let-7a between controls and patients with asthma. | 24 |
| MIR155 | In miRNA-155-deficient mice there is an increase in airway remodeling | 15 |
| In patients with bronchial asthma, the level of expression of miR-155 was reduced in comparison with the control group. | 10 | |
| miRNA-133a | A decrease in the level of miR-133 in asthmatic murine models led to an increased activity of RhoA, which in turn leads to an increase in airway contraction | 15 |
| MIR146A | Reduction in the expression of miR-146 leads to inhibition of target genes involved in the Th1 response | 11 |
| MIR21 MIR126 | The expression of miR-21 and miR-126 in bronchial epithelial cells was increased in both groups of patients (one group of patients who received treatment and the other who did not). At the same time, expression of both miR-21 and miR-126 was significantly higher in the group that did not receive treatment than in the treatment group | 30 |
Many people all over the world suffer from allergies. Allergic diseases, including asthma, are part of a group of heterogeneous diseases. Therefore, individual methods of treatment are especially necessary for allergic diseases that are hard to cure. In recent years, regulatory miRNA has attracted the great attention of scientists. The role of these regulatory molecules in the mechanisms of the development of allergic diseases is just starting to be studied. In spite of the fact that the miRNA functions in the pathogenesis of asthma are at the initial stages of the study, several miRNAs have been identified that can potentially promote or suppress development of asthma. It is very important to continue exploring miRNA functions, because these molecules act as a potential target for the targeted therapy of allergic diseases. In addition, miRNAs can potentially be non-invasive biomarkers. In this review, we presented miRNA, the role of which in asthma is somewhat already studied. Further research is necessary to fully assess the role of miRNA in the mechanisms of asthma development, as well as the use of these molecules in new methods of treatment allergic diseases.
Conflict of interestThe authors have no conflict of interest to declare.