INTRODUCTION
Juvenile idiopathic arthritis (JIA) is the most common rheumatologic disease in the pediatric population. According to a national study, the estimated incidence in Chile is 5.6 cases per 100,000 children under fifteen years of age1. Presentation, clinical course, laboratory parameters, and response to therapy may vary widely. It has been necessary to classify the disease in subgroups in order to identify those patients whose prognosis and response to therapy might be different2.
Most patients respond to conventional management with non-steroidal anti-inflammatory drugs (NSAIDs) and disease-modifying anti-rheumatic drugs (DMARDs), especially if administered early during the course of the illness. These agents have both, improved disease prognosis and allowed steroids doses to be reduced, consequently reducing their associated side effects.
However, a significant number of patients in the polyarticular JIA subgroups or systemic JIA with polyarticular involvement experience severe and aggressive disease resulting in erosive arthritis, joint destruction, functional disability, growth retardation and unresponsiveness to treatment, even when begun early. The persistent disease activity compounded by the cumulative side effects of long term medication has a significant impact in emotional and psychological development, quality of life and family dynamics. For these refractary groups, the use of biological therapies such as tumor necrosis factor alpha (TNFα) antagonists has been suggested3-5. These biological agents have shown therapeutic efficacy in Rheumatoid Arthritis (RA). They modify its course and prognosis by blocking the inflammatory activity of TNFα. This produces a down regulation of the inflammatory response resulting in decrease proliferation of synoviocytes, collagenase production, cell-adhesion molecules expression, bone resorption and cartilage destruction.
The purpose of this study is to report our clinical findings on the use of infliximab, a monoclonal antibody that neutralizes the pro-inflammatory action of tumor necrosis factor alfa (TNFα), in pediatric patients suffering from JIA refractary to conventional therapies.
PATIENTS AND METHOD
Four children with the diagnosis of JIA, followed up at the Immunology Unit of the Exequiel Gonzalez Cortes Hospital were included: two girls had systemic JIA with polyarticular involvement; one had seronegative polyarticular JIA and one boy had JIA associated to enthesitis.
Infliximab (Remicade®, Schering Plough, USA) was administered through a continuous infusion pump at dosages ranging from 2.5 to 2.9 mg/Kg/ dose (median 2.75), at weeks 0, 2 and 6. Subsequently, they received the drug every 8 to 10 weeks. During the first year, patients were admitted to the hospital for treatment for a period of 24 hours. They were kept in isolation and monitored hourly. Later on, because no adverse reactions developed, hospital stay was reduced to six hours.
The following parameters were evaluated at week 0 and at the end of the follow up period: number of joints with active arthritis, number of joints with limited range of motion, physician global evaluation of disease activity and parent assessment of child's overall well-being (rating 10 for maximum disease activity and 0 for no disease activity). Likewise, pain rating by the patient was done using a visual analogue scale. Serum measurements of acute phase reactants were obtained. The definition of improvement of RA by the American College of Rheumatology (ACR 30)6-8 was applied. This is based in the overall assessment of decreased disease activity done by a physician; overall assessment of disease activity according to the patient or his/her mother, functional ability, number of joints with active arthritis, number of joints with limited range of movement and ESR.
Improvement of at least 30 % from baseline from the beginning of the new therapy in 3 of the 6 variables included and not more than one variable worsening by more than 30 % was considered significant. In the overall assessment, a maternal evaluation was included because the patients were children. Furthermore, it was considered of clinical interest to assess morning stiffness in minutes and reduction or suspension of steroid and methotrexate.
The t-test for paired samples was employed to compare the changes in JIA parameter for each patient between entry and end of treatment with infliximab. Results are expressed as mean and standard deviation; a value of p < 0.05 was considered statistically significant. The protocol had the approval from the local Ethics Committee and full informed and signed consent was obtained from parents.
RESULTS
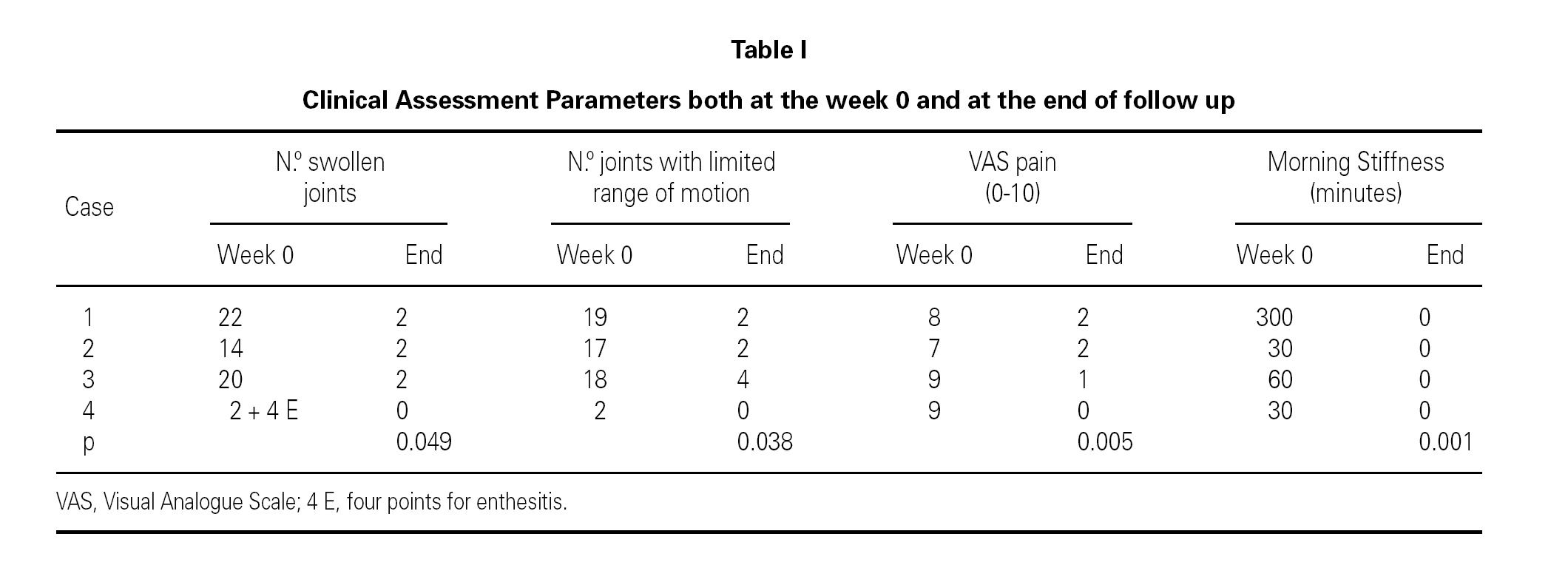
Four patients, three girls and one boy, ranging from 10 to 16 years of age, with 1 to 9 years of disease history, were included in the study. They had been taking NSAIDs, oral steroids (0.2 to 0.3 mg/kg/ day prednisone equivalent), and methotrexate (10.8 to 12.7 mg/m2/week), subcutaneously. The patient with JIA associated to enthesitis was also taking mesalazine (Salofalk®) for Ulcerative Colitis and beta-agonists plus inhaled steroids for Bronchial Asthma. The conventional therapies had proven ineffective in all four patients, whose inflammatory activity, functional limitations and poor quality of life were persistent. The duration of treatment was 22 months on average (range 11 to 33 months) at the time of this report. Table I shows the number of swollen joints, the number of joints with limited range of motion, morning stiffness in minutes, and pain assessment on the Visual Analogue Scale at the beginning and at the end of the follow-up period. All variables showed a statistically significant decrease by the end of the study period.
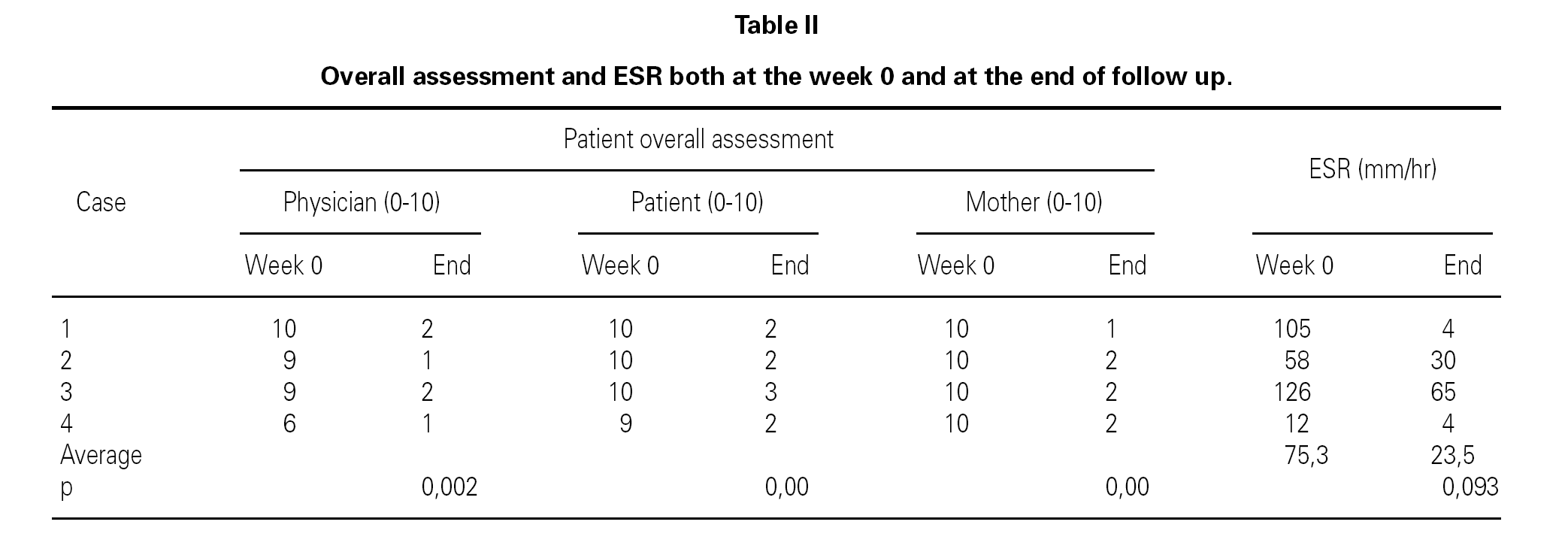
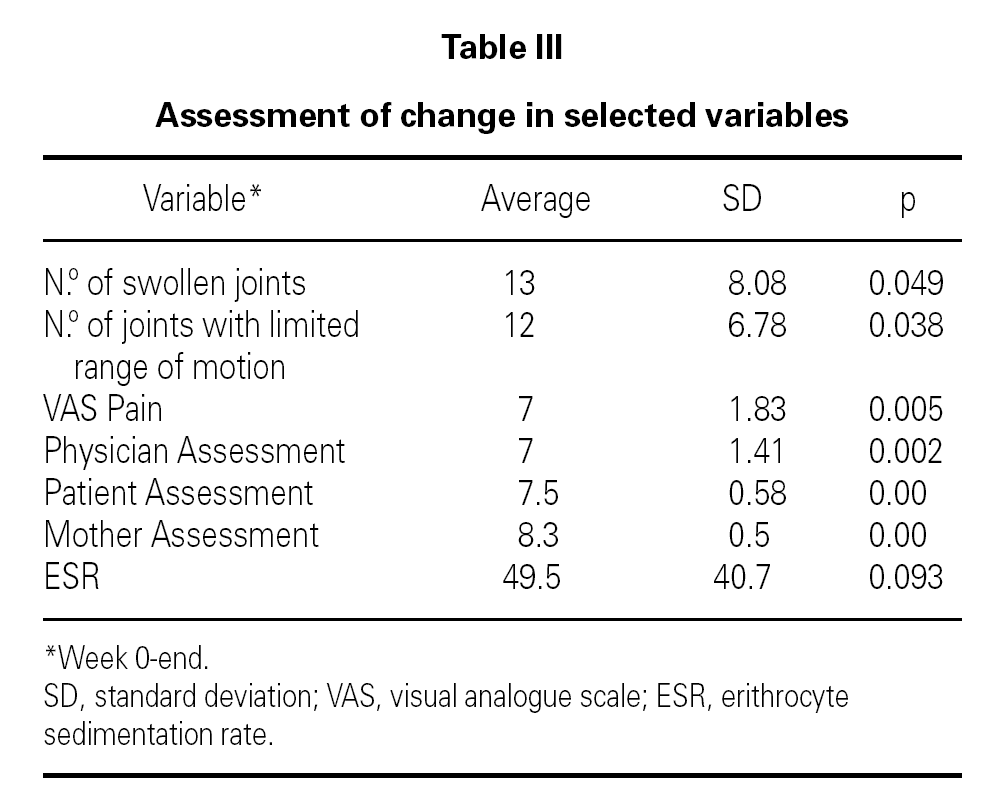
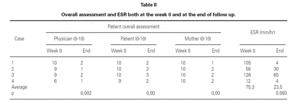
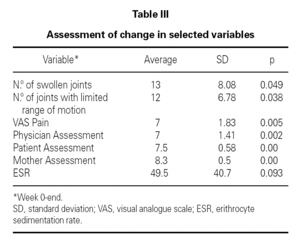
Table II shows the physician, patient and mother evaluations. ESR at baseline and at the end of the follow-up period is also shown. All clinical parameters showed a statistically significant reduction at the end of the follow-up period. ESR also decreased in all patients, although the levels did not reach statistical significance (table III).
All four patients treated with infliximab had a good response, including a drop in ACR 30 activity indexes. In addition, a reduction of morning stiffness and an average reduction of 41.7 % in oral steroid dosages were shown. All patients received subcutaneous methotrexate at the start of biologic therapy; in two cases this treatment was discontinued at the end of the follow-up period because the disease was considered clinically inactive.
Neither adverse reactions nor severe infections were seen in this series. Parents also agreed that the quality of family life had improved.
DISCUSSION
Based on standardized clinical and laboratory parameters of disease activity we have shown that this group of pediatric patients suffering from JIA refractary to conventional therapies had good response to infliximab treatment and a consistent improvement of their quality of life.
One patient (case 1) began therapy at 16 years of age, after nine years of clinical disease activity and evidence of radiological progression. The disease course showed erosive polyarticular involvement, more predominant in the lower extremities. As soon as clinical and laboratory inactivity was shown, hip and knee arthroplasties were planned. We emphasised the improvement of functional ability from Steinbrocker grade IV to II for RA.
The case 2 was a 13-year-old patient with a five-year course of systemic JIA involving axial and several peripheral joints. She had a history of two macrophage activation events which required intravenous immunoglobulin and high doses steroid. All this lead to secondary Cushing syndrome and stunted growth. After 19 months of treatment with infliximab, a significant reduction of inflammatory articular scores and steroid dose was obtained with no evidence of macrophage activation.
The third patient (case 3) was a girl with systemic onset of JIA at the age of 9 with a rapidly progressive polyarticular disease and grade III functional ability at the start of infliximab therapy. Treatment began one year after the start of the disease and after 26 months of treatment, the patient has improved to grade I functional ability and was on low doses of oral steroids without methotrexate.
Finally, a boy (case 4) diagnosed with active Ulcerative Colitis and Bronchial Asthma, refractary to conventional high-dose treatments. He had active and progressive JIA with functional limitations, morning stiffness and ankle arthritis associated to enthesitis which restricted both, daily and school activities. After eight months of infliximab administration, methotrexate was successfully discontinued; no reactivation of Bronchial Asthma was seen, therapy for Ulcerative Colitis remained unaltered and prednisone doses were reduced. Four months after discontinuing infliximab treatment, the patient is still rheumatologically inactive.
Better knowledge of RA pathogenesis, especially JIA, in addition to the concept of immune-mediated diseases; have brought about changes in the therapeutic schemes. TNFα is a fundamental cytokine in joint inflammatory response; in fact, high TNFα concentrations are found in the synovial fluid of these patients. TNFαis produced by cells located at the cartilage-pannus junction and may affect chondrocyte metabolism through cartilage destruction9. The role of cytokines, especially TNFα, has been extensively studied10-12. It has been shown that interleukin 1 (IL-1), IL-6 and TNFα play a role in inflammatory activity and stimulate both synovial cell proliferation by inducing production of collagenase and bone resorption. Moreover, it triggers the release of adhesion molecules. TNFα binds to one of two cell membrane receptor sites (p55-p60 or p75-p80), causing joint inflammation, cartilage destruction and various other clinical manifestations of the disease13.
Two pharmacological mechanisms have been used to inhibit TNFα activity: one works through the development of anti-TNFα monoclonal antibodies like infliximab (a macromolecule composed of human IgG and a fraction of murine origin that constitutes the union site of TNFα) and adalimumab (Humira®, a human monoclonal antibody which is similar both in composition and pharmacological action). The other mechanism used to inactivate TNFα is to block the soluble receptor, as the case of etanercept (Enbrel®); in this case, receptor molecules separated from the cell surface act as competitive TNFα inhibitors.
The availability of TNFα antagonists for clinical use has modified the therapeutic schemes even in early stages of RA, as reported with the use of infliximab combined with methotrexate, which inhibited the progression of structural damage during a two-year treatment period, pointing to a potential long-term benefit by preventing the progression of radiological joint damage14.
In RA, improvement has been shown in therapeutic response indexes based on ACR criteria6. The use of biological action drugs combined with methotrexate has accomplished better therapeutic responses and improvements in immunological tolerance than the administration of the anti-TNF therapy alone. The ATTRACT study (Anti-TNF Therapy in Rheumatoid Arthritis) showed that patients with active RA who received infliximab and methotrexate had augmented clinical and radiological benefits at 30, 54 and 102 weeks15.
Collaborative studies in JIA using etanercept have been conducted with promising results16. An open multicentric collaborative study assessed etanercept safety and efficacy in children older than four years of age with active polyarticular-type disease and small tolerance or inadequately responsiveness to methotrexate. Responders were included in a double-blind study where either placebo or etanercept were given for a period of four months or until relapse was seen. The responders were defined as those individuals showing at least 30 % improvement in three out of six indicators of disease activity. They concluded that etanercept caused significant disease improvement with good drug tolerance.
In another prospective, non-randomised, open study of children over 3 years of age with refractary polyarticular JIA17, infliximab was given to 14 patients and etanercept was administered to 10 patients. DMARDs were also used. There was significant improvement in both groups of patients, allowing for a reduction or discontinuation of DMARDs in selected cases. We had a similar experience with our patients.
During our follow-up period, with infliximab administered at regular intervals for a minimum of eleven months, patients showed clear improvement according to current ACR criteria, better than ACR 30, with no adverse effects or infections. At present, they are rheumatologically inactive and autonomous in their daily life.
From this experience, with the use of infliximab in four pediatric patients with refractary JIA, we found a significant improvement in clinically status and quality of life. Furthermore, no adverse effects were seen in our patients. We believe that our results add information on the effectiveness and safety of Infliximab as previously reported, although multicenter clinical trials are still needed18. We expect than in the near future, the administration of biological therapy could be indicated earlier during the course of the disease, mainly in rapidly progressive cases and also in refractary forms of JIA.
ACKNOWLEDGMENTS
We thank to Dr. Alejandra Aird her technical assistance.
Correspondence:
Prof. Arnoldo Quezada L.
Enrique Matte 1525 San Miguel
Santiago de Chile
Phone-Fax: + 56-2-5557006
E-mail: aquezada@med.uchile.cl
E-mail: caromah@hemo.unc.edu.ar