INTRODUCTION
Allergen specific immunotherapy (SIT) is an effective form of treatment in allergic respiratory diseases. It was first used in medical practice by Noon at the beginning of 1990's1. Since then many studies have been conducted to show its efficacy and safety. WHO Position Paper and EAACI Position Paper have carefully revised the literature and concluded that SIT performed by injections or by local (nasal, sublingual) is effective and safe under adequate conditions2-4. In 2000, a meta-analysis assessing the efficacy of SIT in asthma including 54 clinical trials has been published confirming the previous reports5.
During the last decade, studies have demonstrated the preventive effect of SIT to reduce onset of new sensitizations to non-related allergens and to improve long term effects6-8. There are only few studies about the duration of the preventive and theurapetic effect of SIT after discontinuation8-10.
The aim of our study was to demonstrate the long term efficacy of SIT in allergic respiratory diseases in childhood and determine whether SIT prevents development of new sensitizations in sensitized children.
MATERIALS AND METHODS
Selection of Patients
107 patients aged between 7-12 years suffering from intermitant asthma sensitized to HDM or grass mix were enrolled in the study. Of these 107 patients; 56 received SIT (43 house dust mite IT and 13 pollen IT). 51 patients treated with medication were included as the control group. Patient records were overwieved. The clinical status, skin prickt test results and pulmonary functions were evaluated for the comparison of three periods of treatment; initial, end of the treatment and five years after the treatment has been ended.
Included patients had the following features:
1. Clinical history of mild to moderate persistent bronchial asthma;i.e patients with asthmatic symptoms > 2 times a week to daily, exacerbations could effect daily activities and sleep, night-time symptoms > 2 times a month, PEF > 80 % predicted with a variability < 20 %.
2. Clinical history of allergic rhinitis.
3. Monosensitization to either Dermatophagoides farinae, Dermatophagoides pteronyssinus and/or grass pollen species.
4. A positive skin prick test result to a biologically standardized depot preparation of HDM (Dermatophagoides pteronyssinus and/or Dermatophagoides farinae) extract or grass polen species.
5. Patients who came to control visits regularly during this period.
6. Patients who had discontinued SIT.
Patients with the following criterias were not included in the study:
Patients who had taken SIT during the previous years.
Skin test positivity to food allergens or inhaled allergens other than HDM or grass pollen mix.
Other diseases that contraindicated SIT.
All patients had been followed with a medical file. Respiratory symptoms and drug intake were recorded in the files. SIT and control group were matched for age, asthma and/or allergic rhinitis severity, respiratory function. During the follow up, all symptoms occuring because of asthma and/or allergic rhinitis had been recorded in the clinical file. Patients had been invited for control visit every three months regularly (After the therapy has been started, patients who had exacerbation symptoms had been evaluated without waiting three months. They have been followed for five years after the treatment has been discontinued.
In every visit; parents and patients had been asked for the following symptoms:
Shortness of the breath with wheeze.
Cough due to bronchial asthma.
Exercise intolerance.
Sneezing, blockage, rhinorrhea of the nose.
Itching,redness,tears and swelling of the eyes.
SIT had been continued for four years. All of the patients who had discontinued the SIT fo four years were included in the study.In the control group; patients were given inhaled Fluticasone dipropionate (400 mg/day) regularly and Salbutamol (600 mg/day) in the exacerbation period. If no improvement was observed with these therapies then prednison (1 mg/kg/day) were given to these patients for one week in the attacks. Pulmonary function tests were performed in the appropiate patients.
Skin tests were repeated by using the same allergen panel 5 years after the discontinuation of the therapy. Pulmonary function tests were also repeated by the same technique to each patient.
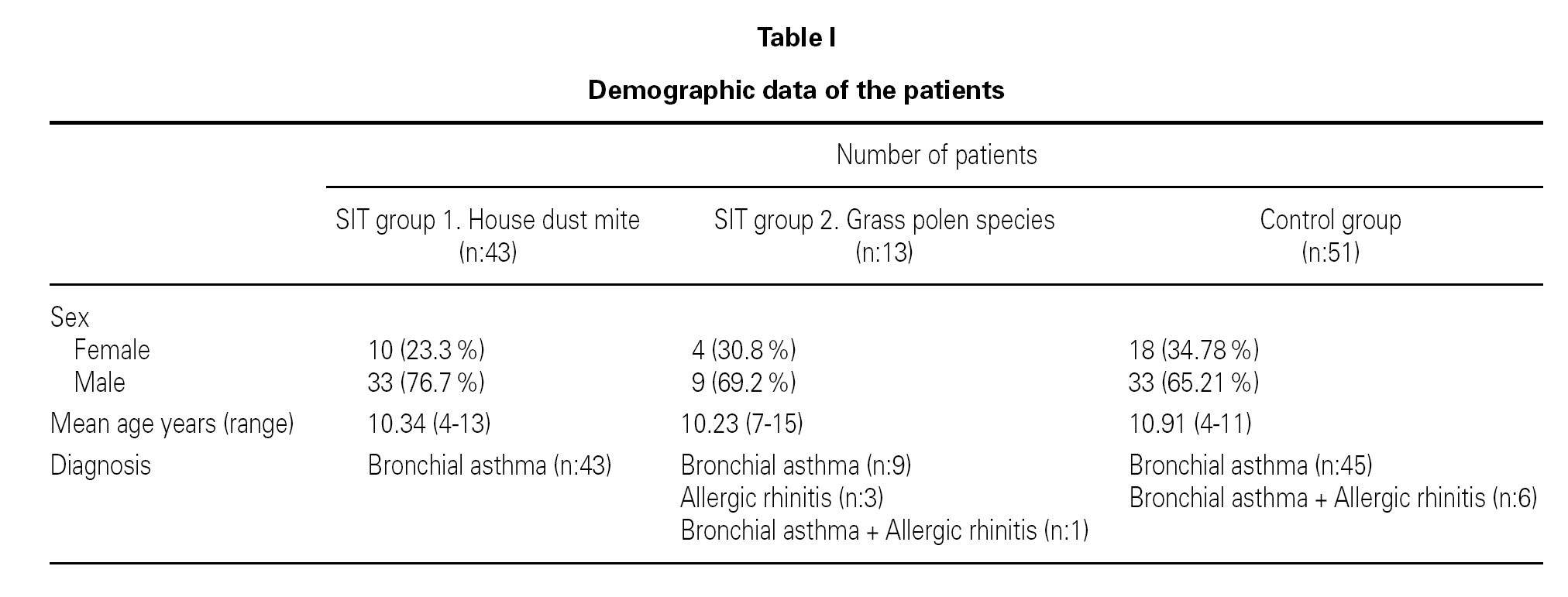
The study group consisted of 43 patients who had sensitizations to house dust mite and 13 to grass pollen species. The control group consisted of 51 patients who were only treated with pharmacotherapy. Both groups were comparable in terms of age and sex. Patient's initial age' median value was; 10.34 years (range, 4-13 years) in the house dust mite group, 10.23 years (range 7-15 years) in the grass pollen group and 10.91 (range 5-12 years) in the control group. Sixty percent of the immunotherapy group and 65 % of the control group were male (table I).
In the house dust mite IT group, all of the patients had bronchial asthma. In the grass pollen IT group, 9 patients had bronchial asthma, 3 had allergic rhinitis and 1 had both bronchial asthma and allergic rhinitis together. In the control group, forty-five patients had bronchial asthma and 6 had both bronchial asthma and allergic rhinitis together (table I).
Skin prick test
Skin prick test was performed by an allergolocist at the time of diagnosis and 5 years after the therapy has ended. It was performed on the volar surface of the forearm according to the European Academy of Allergology and Clinical Immunology (EAACI) recommandations4 with a panel of standardized allergen extracts (including Dermatophagoides pteronyssinus, Dermatophagoides farinae, 10 mg/ml histamine as positive control and a negative control). All patients had been guided not to take any drugs one week before the skin prick test.
The perimeter of the resulting wheal calculated according to the formula after 15 minutes (D + d)/2, where D is the maximum diameter and d is the perpendicular diameter at the midpoint). A mean diameter > 5 mm was considered as positive reactivity. Skin prick test reactivity (mean diameter) was compared before and after the treatment in each patient.
Immunotherapy Protocol
SIT was performed with a standardized depot preparation of house dust mite mix (Dermatophagoides pteronyssinus, Dermatophagoides farinae) and grass pollen mix (Phostal, Stallergenes France).
Extract administration was directed according to the EAACI indication8
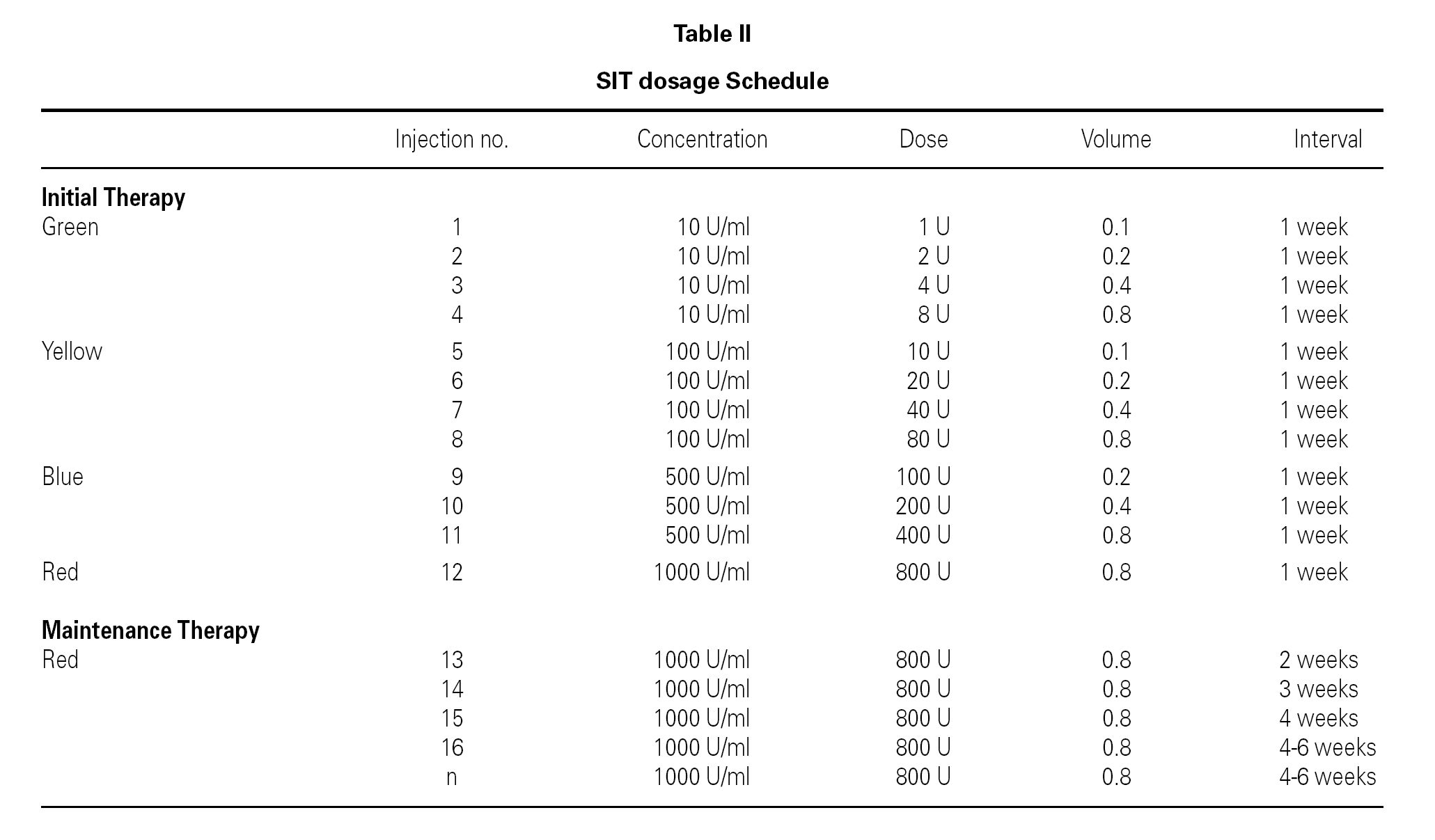
Extract doses were gradually increased up to the maximum tolerated dose (table II). Doses were arranged according to the clinical state and lung function results as suggested by the EAACI position paper (4). All the patients have received SIT for the same time period for four years between years 1996-2000.
Pulmonary function test
Pulmonary function tests were performed with a spirometer (Sensor Medics V max 20 C). Patients performed at least three lung function tests to obtain best and FEV1, FVC, FEF25-75 and PEF values. Flow volume curves were performed in all patients before the therapy and after the discontinuation of the therapy protocol.
Pulmonary function test was performed in 41 out of 56 patients in SIT group. We were not able to perform pulmonary function test in 15 patients because we had been not able to obtain a reliable forced expiratory maneuvre either due to their age or failure in cooperation. For the same reasons, we had been able to perform pulmonary function tests in 39 out of 51 patients in the pharmacotherapy group.
Statistical Analysis
Statistical analysis was performed by using SPSS 11.0 for Windows. Quantitative outcomes were expressed with medians. A Mann-Whitney U rank, Kruskal-Walis H test, Student' t test was used for comparison between groups. The main outcome measurement was compared by means of a Chi square test. A significance level of p < 0.05 was used.
RESULTS
Clinical Evaluation
In the IT group, 54 of 56 patients had one or more of the symptoms; cough, wheeze, dyspnea, rhinitis, conjunctivitis before immunotherapy. After the SIT has been discontinued 29 patients still had one of these symptoms. At the end of 5 years, 44 patients had no symptoms. One or more of the symptoms mentioned above were still lasting in only 12 patients. All of the patients in the medically treated group had one or more of these symptoms before the treatment. At the end of the medical treatment 33 of 51 patients had no symptoms. There was a significant decrease in the number of patients with symptoms in both groups both just after the treatment and five years after the treatment.
Evaluation of sensitization
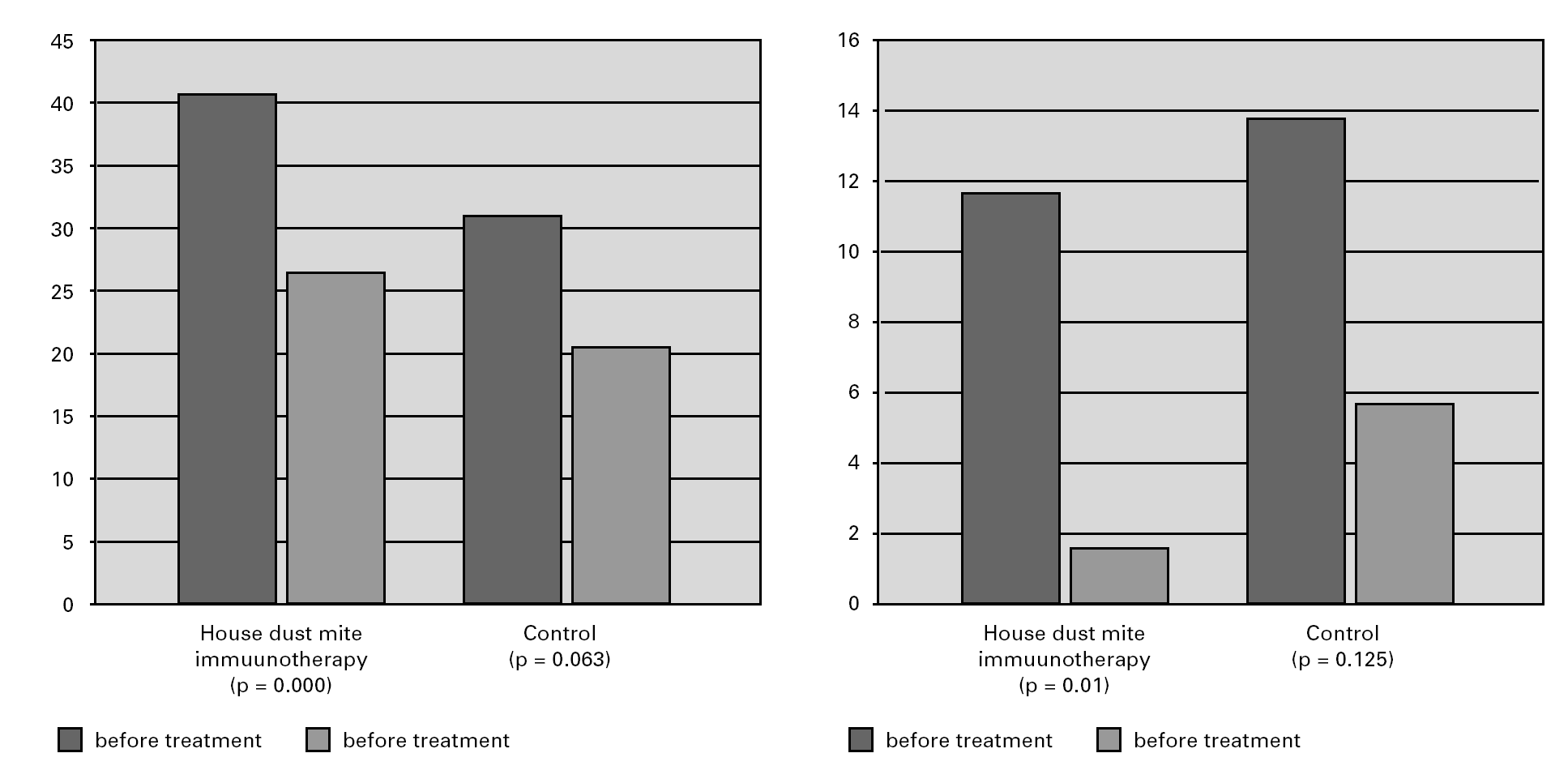
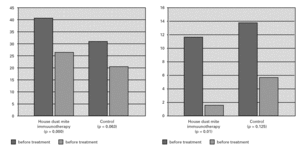
Wheal size in response to skin prick testing with allergens was compared in each patient before and after the treatment. Reevaluation of prick test sensitivity five years later revealed that the immediate skin reactivity to house dust mite or grass pollen species remained significantly lower in the SIT group(both house dust ite and grass polen species) than the control group(p < 0.005) (fig. 1).
Figure 1.—Skin prick test reactivity (mean diameter) showed a statistically significant decrease in SIT patients. Non parametric Mc Nemartest was used for statistical analysis.
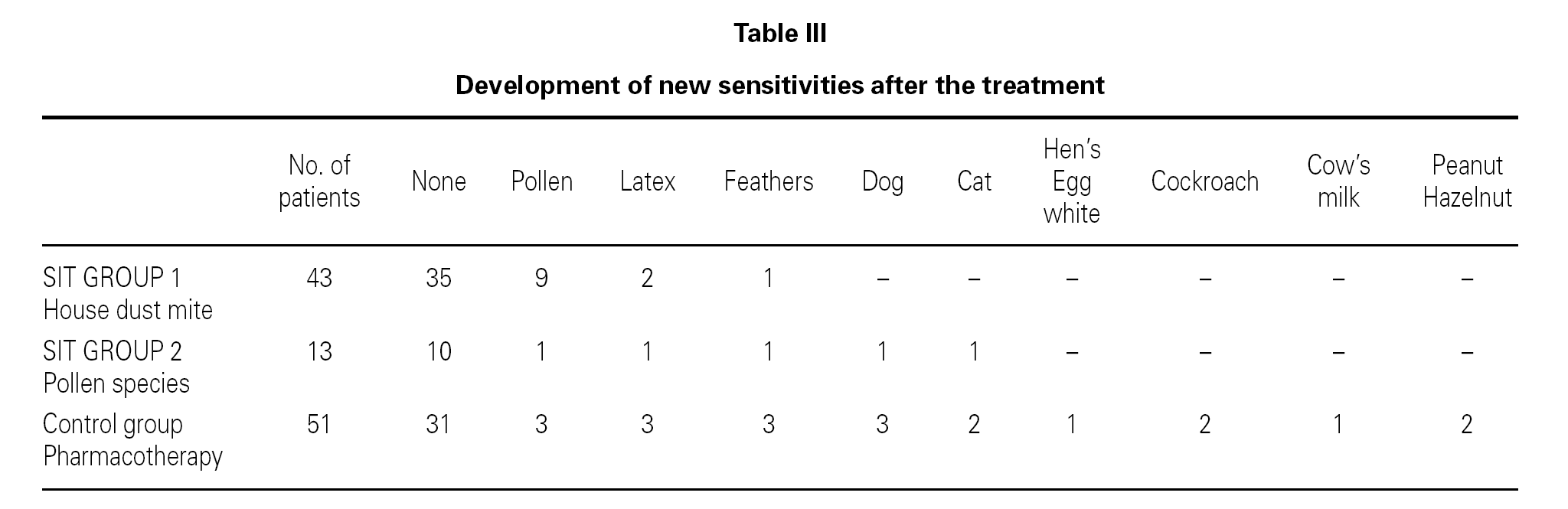
At the end of the study period, 35 of the 43 (81.39 %) patients in the house dust mite IT group, 10 of 13 (76.92 %) patients in the grass pollen IT group showed no new sensitizations. However 20 of the 51 (53.84 %) patients in the control group showed new sensitizations (table III). Children who received SIT had less likely developed new sensitivities to inhalant allergens than those who did not receive specific immunotherapy. The comparison of both house dust mite and grass pollen SIT groups and control group for this parameter was statistically significant (p = 0.033)
The most frequent new sensitizations at the end of the study were against grain pollens, grasses, followed by feathers and latex (table III). Two patients have developed cat and dog dander allergy. Hazelnut, walnut and peanut allergy was seen in one patient.
Pulmonary function test
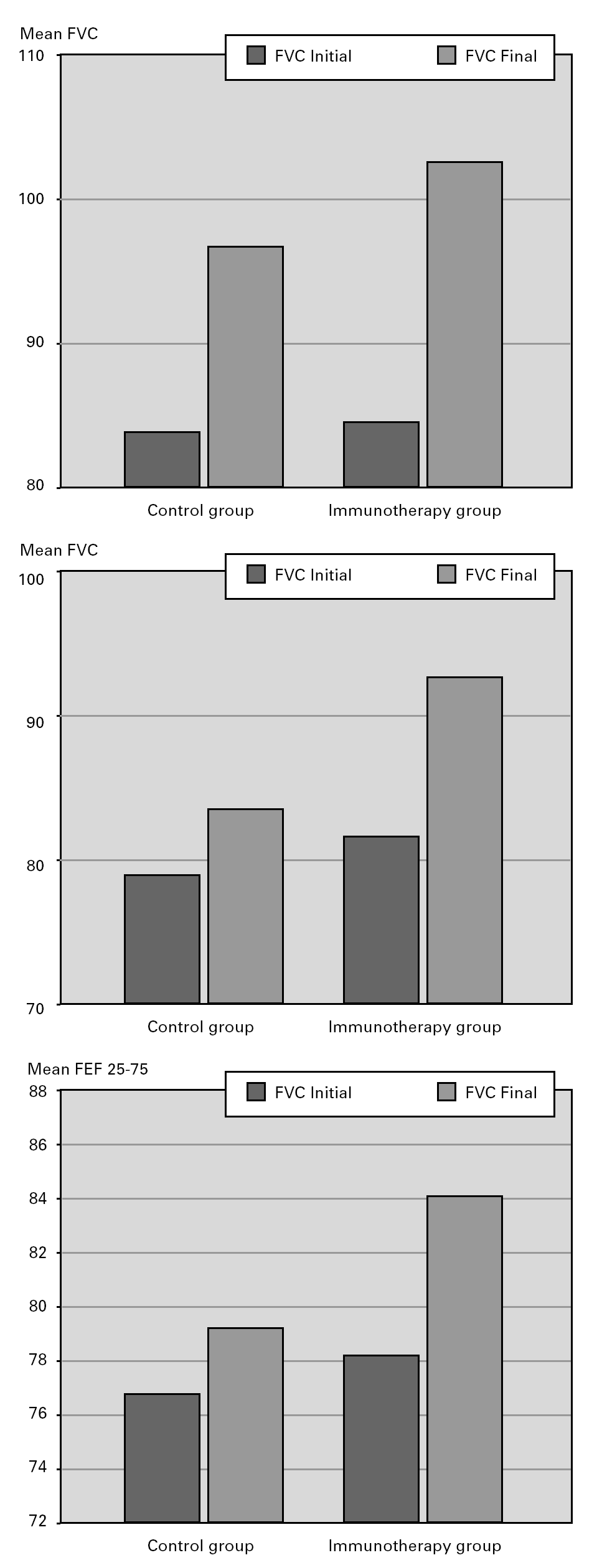
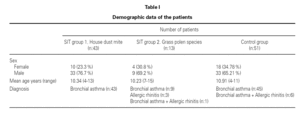
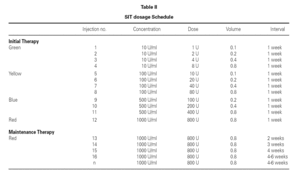
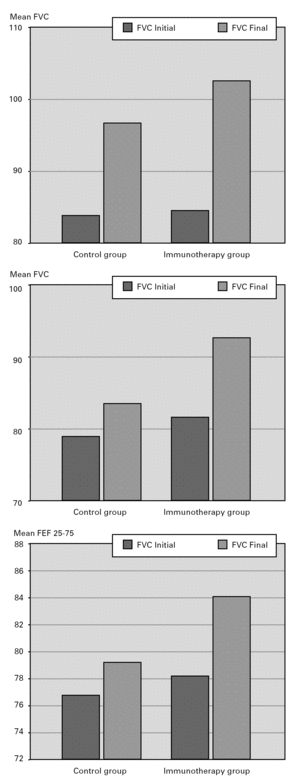
When patients in the immunotherapy and control group were compared for pulmonary function tests, there was statistically significant improvement in the values other than PEF (FVC, FEV1, FEF25-75) in the SIT group after the treatment, independently of the lung growth effect but we did not observe the same improvement in the control group (P < 0.05) (fig. 2).
Figura 2.—Lung function measurements (predicted values meanpercent) in SIT subjects vs. controls. A:for FVC; B:for FEV1;C:forFEF25-75
DISCUSSION
Immunotherapy consists of regular injections of progressively increasing doses of relevant allergens to sensitive patients. Since it was first described in 1911 by Noon1 and Freeman11, allergen specific immunotherapy has been widely used and several double-blind placebo controlled studies have revealed its safety and efficacy in reducing symptoms evoked by allergen.
In this study, for the evaluation of the long term efficacy of SIT and it's role in the prevention of new sensitizations; we compared the baseline complaints,skin prick test results and pulmonary function test values with the ones after the tretament has been discontinued.
In our study, there was a significant decrease in the number of patients with symptoms of both the patients receiving SIT and the control group. In contrast to our results, Pifferi et all12, reported that there was a more significant decrease in asthmatic symptoms in the SIT group than the control group. Another study performed by Walker et al13 demonstrated that a specific immunotherapy with grass pollen extracts could reduce seasonal asthma symptoms. In a meta-analysis performed by Abramson et al5, the authors confirmed that allergen SIT can significantly reduce asthma symptoms. Eng Pa et all; examined their patients to investigate whether there is a prolonged benefit 12 years after SIT is stopped. Total hay fever symptom score (P < 0.03), use of medication (P < 0.05), and combined symptom and medication score (P < 0.03) remained lower in patients with previous SIT when compared with the control group. Decreased immediate skin response to grass pollen returned 12 years after cessation of SIT. The percentage of new sensitization, however, was significantly smaller in patients with previous SIT (58 %) compared with the controls (100 %, P < 0.05) There was a tendency for lower prevalence of seasonal asthma in the post-SIT group9.Our study shows that, when appropiately used, medical treatment can also provide improvement in symptoms as well as immunotherapy does. The role of drugs in the treatment of respiratory diseases cannot be under estimated and it has already been shown that the improvement obtained with SIT can be enhanced if corticosteroids were also given14.
The incidence of specific sensitizations to indoor and outdoor allergens is high in the first decade of life. After a sufficiently long period, Ig E antibodies are produced and children will convert from skin negativity to positivity. On the basis of skin testing, infants are sensitized to food allergens in the early life period. Also, molds and danders give rise to early sensitizations.Mite sensitivity appears later in life and pollen sensitivity is the last allergy to develop15-19. Nevertheless, this course is not always followed and all scenarios are possible. In our study, the median age in the house dust mite and pollen alergy groups was 10.34 and 10.23, respectively which is consistent with the course defined above.
It has been shown that SIT has possible actions on the regulation of Th1: Th2 balance, lymphocyte function and responsiveness to the allergen, and the production of cytokines and IFN-γ20-24. SIT has also been shown to significantly decrease the production of IL-4 and IL-520,22,23, increase production of IFN-γ20,22-24 decrease the number of inflammatory cell in the nose21,25, and decrease the number of mast cells in the skin26. These actions may alter the ongoing allergic and inflammatory response, and therefore modify the natural course of Ig E mediated disease of the respiratory tract. The mechanisms that explain the lower rate of new sensitizations in children given SIT is still unclear. A decreased release of the mediators of inflammation could reduce bronchial hyperreactivity and prevents the activity against the development of new sensitizations27-29. In 1997 Des Roches et al27 made a prospective non randomized 3 year follow-up study in the asthmatic children under the age of 6 years, who are only allergic to house dust mites. 22 children monosensitized to house dust mites receiving specific immunotherapy, were compared with a group of 22 age matched controls. Approximately 45 % (10 out of 22) of the children receiving immunotherapy did not develop new sensitivities, whereas none of the children in the control group remained free of new sensitivities measured by skin prick testing and by the measurement of allergen specific immunoglobulin E antibodies. They revealed that during allergen injection immunotherapy the likelihood of developing new sensitivities which had not been observed at baseline was significantly reduced in the immunotherapy group compared with the control group. Pajno et al24 have studied 134 children who had intermittant asthma with or without rhinitis who are sensitized only to mite allergen as demonstrated by skin prick test. Seventy five of these children received specific immunotherapy with a mite allergen extract and rest served as controls. IT was given for 3 years. After an observation period of six years, more than 75 % of the specific immunotherapy group showed no additional sensitization compared with 33 % in the control group. Their results indicated that, significantly fewer children in the immunotherapy group developed new Ig E mediated sensitizations compared with the control group. In 2001, D'Ambrosio et al30 studied on 8396 subjects monosensitized to mites, grass, olive, Compositae, Corylacea-Betulaceae or Parietaria, retrospectively. 7182 patients received specific immunotherapy for 4 years and 1214 patients were treated with drugs during this period. They have shown that that specific immunotherapy for 4 years significantly reduced new sensitizations in the monosensitized patients suffering respiratory allergic diseases. In our study, patients treated with SIT showed a significantly lower rate of new sensitizations (19.64 % vs. 53.84 %) This is in agreement with the literature; suggesting that immunotherapy prevents the onset of new sensitizations.
Our lung function test results were in contrast with the literature. In the meta analysis by Abramson et al4, it has been demonstrated that SIT had no consistant effect on lung function. However in the SIT group, we observed significant improvement in respiratory functions at the end of the study. We think that; the improvement in the lung function results in the SIT group was due to the significant reduction in non specific bronchial hyperreactivity as previously reported31,32.
SIT applied by subcutaneous injections of allergens is the only causal treatment controlling the disease activity and providing symptomatic improvement long after discontinuation. In our study, in addition to the prevention of development of new sensitivities, the severity of house dust mite and pollen allergy skin prick test reactivity demonstrated by the wheal size has reduced by SIT treatment, in aggrement with the literature.
CONCLUSION
In conclusion, our data confirmed that administration of specific immunotherapy in allergic patients significantly reduced symptoms and development of new sensitizations and improved lung function tests. Existing allergen sensitivity diminished or disappeared after specific immunotherapy. We suggest to apply specific immunotherapy to house dust mite and pollen allergy to relieve symptoms and give a long lasting effect. SIT modifies the natural course of the illnessby improving the clinical outcomes and prevents new sensitization. Despite these encouraging data, prevention of new sensitizations by SIT requires further evaluation.
Correspondence:
Ebru Arhan
Yunus Emre Cad. Yig(breve)itler Sok.
No: 9/3
Incirli
Ankara, Turkey
Phone: + 90-312-3232995 + 90-312-2026044
E-mail: petekarhan@yahoo.com.tr