The prevalence of allergic bronchopulmonary aspergillosis (ABPA) in patients with bronchial asthma remains unknown. We evaluated the roles of various laboratory tests in the diagnosis of ABPA, including, skin prick test (SPT) for Aspergillus fumigatus (Af), and serum Af specific IgE and IgG antibody measurement.
MethodsA total of 50 asthma patients with more than 1000cell/μL of peripheral blood eosinophils were prospectively collected between January 2007 and September 2011. Evaluations using SPT for Af, serum total IgE and specific IgE antibody to Af by CAP system, IgG antibody to Af by enzyme immunoassay (EIA) or CAP system were performed according to the essential minimal criteria for the diagnosis of ABPA – asthma, immediate cutaneous reactivity to Af, elevated total IgE, and raised Af specific IgE and IgG.
ResultsAmong 50 patients, three patients (6.0%) were diagnosed as ABPA, of whom each confirmed five items of the essential minimal diagnostic criteria for the diagnosis of ABPA. Six patients (12.0%) showed negative responses to Af in SPT, but positive responses in specific IgE by CAP system. Eight patients (16.0%) showed negative responses to IgG to Af by CAP system, but positive responses by enzyme immunoassay (EIA).
ConclusionsSPT and serum IgE to Af measurement by CAP system should be performed simultaneously. It is reasonable to set up cut-off values in Af specific IgE/IgG by CAP system for the differentiation of ABPA from Af sensitised asthma patients.
Allergic bronchopulmonary aspergillosis (ABPA) is a complex hypersensitivity response to the presence of Aspergillus fumigatus (Af) in the bronchial mucosa,1 which includes immediate hypersensitivity reaction (type I), and antigen-antibody mediated reaction in genetically susceptible patients (type III).2,3
Despite the publication of numerous case reports on ABPA, the exact prevalence of ABPA in patients with bronchial asthma remains unknown. This may be due to the lack of reference values within individual serological methods to differentiate between ABPA and Af sensitised asthma patients. Therefore, the condition remains underdiagnosed in many countries, with reports of mean diagnostic latency period as great as 10 years.4 The original criteria for the diagnosis of ABPA included bronchial asthma, immediate skin test reactivity to Af, elevated total and Af-specific serum immunoglobulin E (IgE), pulmonary opacities, central bronchiectasis, peripheral blood eosinophilia and positive serum precipitins (IgG) against Af.5,6 There is also a set of minimal essential criteria for the diagnosis of ABPA7–9 which include asthma, immediate cutaneous reactivity to Af, elevated total and Af-specific IgE/IgG and proximal bronchiectasis on computed tomography (CT) of the chest. Such patients can be designated as having ABPA-central bronchiectasis. In patients with asthma, ABPA is sometimes diagnosed in the absence of the typical proximal bronchiectasis, in such cases, it is designated ABPA-seropositive.9
According to the existing diagnostic criteria, the most important step in the diagnosis of ABPA is to determine the positive immediate cutaneous reactivity to Af in patients with asthma at an early stage. Recently, O’Driscoll reported discrepancies between the results of the skin prick test (SPT) and serum specific IgE to fungal antigens including Af.10
Also, it has been demonstrated that intradermal tests with Af should be performed in order to rule out the sensitisation of Af in patients with asthma.11 However, performing such intradermal tests is time consuming as wells as being quite uncomfortable for the patient, and thus it is not a common practice in clinical settings.12
Detection of precipitating antibodies for Af has some disadvantages. Specificity13 and sensitivity14 are limited, reproducibility is poor, and antibody concentrations cannot be measured. Moreover, this method is time-consuming and therefore not recommended for routine diagnostic purposes.15,16
Furthermore, no reference values specific to IgE and IgG to Af in terms of the differentiation of ABPA from asthma patients sensitised with Af have been generated.
The aim of this study was to determine the prevalence of ABPA in patients with eosinophilic asthma, and who have more than 1000cells/μL in peripheral blood, as well as to evaluate the roles of various laboratory tests in the diagnosis of ABPA including, SPT for Af, as well as serum Af specific IgE and IgG antibody measurement.
Materials and methodsThe present study was a retrospective analysis of prospectively collected data of 50 patients with bronchial asthma from January 2007 to September 2011. This study was approved by and followed the guidelines of the institutional review board of Dong-A University Hospital.
The diagnostic criteria used for bronchial asthma include the presence of reversible airway obstruction or airway hyperresponsiveness (AHR). Reversible airway obstruction was defined as an improvement in the FEV1 of ≥200ml and 12% after administration of two puffs of salbutamol by metered-dose inhaler. AHR was defined as the concentration of methacholine in mg/ml inducing a 20% decrease in FEV1 (PC20 FEV1) of ≤8.0mg/ml. All patients had peripheral blood eosinophilia of more than 1000cells/μL in the initial evaluation. Study patients were classified by the severity criteria according to GINA.17 Patients who had received systemic glucocorticoids for more than three weeks in the preceding three months were excluded from the study.
We prospectively evaluated the following items: allergy SPT with 50 common inhalant allergens including Af (Allergopharma, Reinbeck, Germany), serum total IgE and specific IgE for Af by CAP system (Pharmacia, Uppsala, Sweden), serum IgG antibodies for Af by enzyme immunoassay (EIA) (Greencross Research Lab., Seoul, Korea) and CAP system.
Diagnostic criteria used for the diagnosis of ABPA were incorporated from minimal essential criteria for the diagnosis of ABPA-seropositive,7–9 which included asthma, the values greater than 3+ response to Af on SPT, total serum IgE concentration the values greater than 417kU/L or 1000ng/mL by CAP system, elevated serum IgE for Af greater than 0.35kU/L18 by CAP system, and IgG for Af greater than 35.0mgA/L by CAP system19 or, greater than 12U/mL by EIA (Greencross Research Lab.)
Statistical analyses were performed using the SPSS programme version 15 (SPSS Inc, Chicago, Illinois, USA). All values were expressed as the mean±standard deviation. Correlations between various parameters were evaluated with Spearman's rank coefficients. Statistical significance had been set a priori at P<0.05.
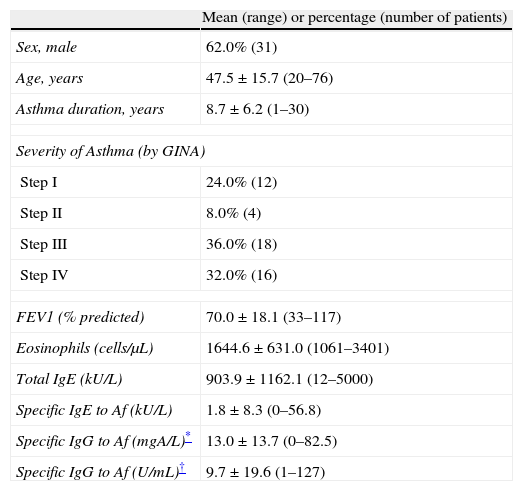
ResultsClinical characteristics of study subjectsA total of 50 patients diagnosed as having bronchial asthma with peripheral blood eosinophilia of more than 1000cells/μL were included in this study (Table 1). The mean age of the study patients was 47.5±15.7 (range, 20–76 years). More than 60% of patients were included in steps 3 or 4 of asthma severity classified by GINA.
Baseline characteristics of 50 asthma patients with eosinophilia of more than 1000/μL in peripheral blood.
| Mean (range) or percentage (number of patients) | |
| Sex, male | 62.0% (31) |
| Age, years | 47.5±15.7 (20–76) |
| Asthma duration, years | 8.7±6.2 (1–30) |
| Severity of Asthma (by GINA) | |
| Step I | 24.0% (12) |
| Step II | 8.0% (4) |
| Step III | 36.0% (18) |
| Step IV | 32.0% (16) |
| FEV1 (% predicted) | 70.0±18.1 (33–117) |
| Eosinophils (cells/μL) | 1644.6±631.0 (1061–3401) |
| Total IgE (kU/L) | 903.9±1162.1 (12–5000) |
| Specific IgE to Af (kU/L) | 1.8±8.3 (0–56.8) |
| Specific IgG to Af (mgA/L)* | 13.0±13.7 (0–82.5) |
| Specific IgG to Af (U/mL)† | 9.7±19.6 (1–127) |
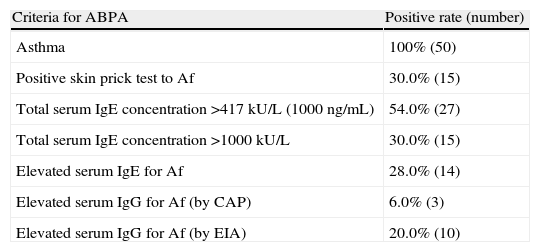
Five diagnostic criteria were used to detect ABPA in asthma patients with eosinophilia (Table 2). The most frequent finding among the diagnostic criteria was total IgE by CAP system, noted in 27 patients (54.0%). However, serum IgG for Af by CAP system was noted in only three patients (6.0%).
Distribution of patients according to the positive rate of each diagnostic criteria.
| Criteria for ABPA | Positive rate (number) |
| Asthma | 100% (50) |
| Positive skin prick test to Af | 30.0% (15) |
| Total serum IgE concentration >417kU/L (1000ng/mL) | 54.0% (27) |
| Total serum IgE concentration >1000kU/L | 30.0% (15) |
| Elevated serum IgE for Af | 28.0% (14) |
| Elevated serum IgG for Af (by CAP) | 6.0% (3) |
| Elevated serum IgG for Af (by EIA) | 20.0% (10) |
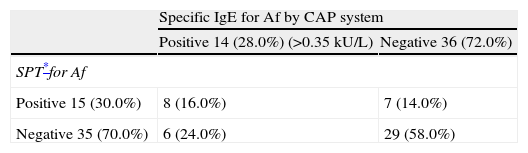
Forty-one percent (21/50) of subjects were sensitised to Af by SPT or CAP systems or both. Among 15 patients with positive responses to SPT for Af, eight patients also showed positive responses to IgE for Af by CAP system. Among 35 patients with negative responses to SPT for Af, 29 of these also showed negative responses to IgE to Af by CAP system, the remaining six patients showed positive responses in specific IgE by CAP system. Overall, SPT and specific IgE tests correlated positively or negatively in 37 (74.0%) patients but 13 patients (26.0%) had disconcordant SPT and specific serum IgE results (Table 3). There were no statistically significant correlations observed between specific IgE for Af and SPT.
Overall results of skin prick test and specific IgE for Af by CAP system.
| Specific IgE for Af by CAP system | ||
| Positive 14 (28.0%) (>0.35kU/L) | Negative 36 (72.0%) | |
| SPT*for Af | ||
| Positive 15 (30.0%) | 8 (16.0%) | 7 (14.0%) |
| Negative 35 (70.0%) | 6 (24.0%) | 29 (58.0%) |
Nine patients showed negative responses to IgG for Af by CAP system, while exhibiting positive responses to EIA. There were statistically significant correlations observed between the results obtained by CAP system and those of EIA (r=0.55, P<0.001).
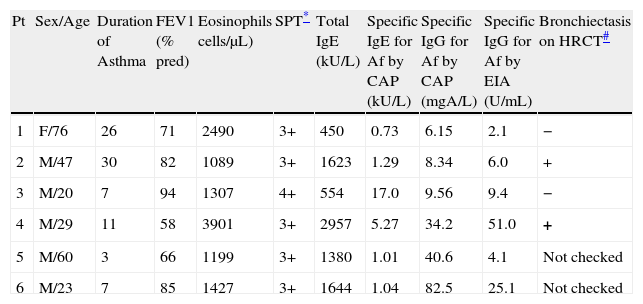
Characteristics of study subjects who met more than four diagnostic criteriaLaboratory findings of patients who met more than four diagnostic criteria are shown in Table 4. There were only two patients who completely met the diagnostic criteria for ABPA. One patient showed a negative response to IgG for Af by CAP system, but a positive response by EIA. Therefore, the overall prevalence of ABPA in this study was 6.0% (3/50).
Clinical, serological and radiological characteristics of study subjects included in more than four diagnostic criteria.
| Pt | Sex/Age | Duration of Asthma | FEV1 (% pred) | Eosinophils cells/μL) | SPT* | Total IgE (kU/L) | Specific IgE for Af by CAP (kU/L) | Specific IgG for Af by CAP (mgA/L) | Specific IgG for Af by EIA (U/mL) | Bronchiectasis on HRCT# |
| 1 | F/76 | 26 | 71 | 2490 | 3+ | 450 | 0.73 | 6.15 | 2.1 | − |
| 2 | M/47 | 30 | 82 | 1089 | 3+ | 1623 | 1.29 | 8.34 | 6.0 | + |
| 3 | M/20 | 7 | 94 | 1307 | 4+ | 554 | 17.0 | 9.56 | 9.4 | − |
| 4 | M/29 | 11 | 58 | 3901 | 3+ | 2957 | 5.27 | 34.2 | 51.0 | + |
| 5 | M/60 | 3 | 66 | 1199 | 3+ | 1380 | 1.01 | 40.6 | 4.1 | Not checked |
| 6 | M/23 | 7 | 85 | 1427 | 3+ | 1644 | 1.04 | 82.5 | 25.1 | Not checked |
This study was performed on a selected population of bronchial asthma patients with eosinophilia, and it was found that the prevalence of ABPA was not more common than had been generally expected.9 Some patients, although showing negative responses to Af through SPT, also demonstrated positive responses to serum specific IgE for Af by CAP system. Furthermore, some subjects who showed negative responses to specific IgG to Af by CAP system were revealed to have positively responded to EIA. These findings suggest that a further consensus on the diagnostic criteria for ABPA, as well as more accurate reference values based on individual laboratory methods, are required for the simpler and more accurate diagnosis of ABPA within clinical settings.
The prevalence of ABPA is believed to be about 1–2% in patients with asthma, and 2 to 15% in patients with cystic fibrosis (CF).9 However, a recent meta-analysis over a 30-year period has shown that the prevalence of ABPA in bronchial asthma was as high as 12.9%.11 The main limitation, which was noted in this review was that all the studies were performed in specialised clinics and may not have been representative of the general population. In this study, the prevalence of ABPA was 6.0% (3 in 50). Subjects of this study were selected among asthma patients who had eosinophilia of more than 1000cells/μL in their peripheral blood. Based on the original major diagnostic criteria,5,6 eosinophilia was recommended as one of the key elements in the diagnosis of ABPA. Furthermore, over 60% of the subjects in this study were classified as being in steps 3 or 4 of severity according to the GINA classification guideline.17 Recently, it has been reported that patients with severe asthma have sensitisations to multiple fungal antigens, and among them Af was the most frequent.10 These findings suggest that subjects of this study were a specialised group who had a high probability of being diagnosed with ABPA. The diagnostic rate of ABPA in these selected patients was, however, no higher than the expected. This finding might be explained by several characteristics of the study subjects. In general, the prevalence of ABPA in patients admitted with acute severe asthma is even higher.20 Additionally, the occurrence of ABPA was significantly higher in patients with acute asthma, when compared to that of outpatient bronchial asthma.21 However, most of our study subjects were outpatient bronchial asthmatics with chronic respiratory symptoms and acute exacerbations. Furthermore, we included asthma patients with eosinophilia to increase the diagnostic probability of ABPA, but this method might paradoxically decrease the diagnostic probability, as some reports18 showed that more than 50% of ABPA occurrence had no associated eosinophilia of more than 1000cells/μL.
Determining the sensitisation status for Af has been regarded as the most important, and first step in the diagnosis of ABPA.22 An immediate cutaneous hypersensitivity to Af antigens is a characteristic finding of ABPA and represents the presence of Af specific IgE antibodies.23 In the case of a negative response in SPT with Af antigen, an intradermal test should be performed to confirm this negativity to the Af antigen.11 However, we postulated that an intradermal skin test with Af antigen might not be adequate for screening to detect the sensitisation status for Af in a real clinical setting. Many patients with ABPA may be minimally symptomatic or asymptomatic, and therefore, the majority of asthma patients should undergo the intradermal test for Af, even though this is more time consuming, labour intensive, and technically complicated. Furthermore, SPT has a high (95%) accuracy in predicting negative results.24 Accuracy for positive results is less, at 50–60%, although these variations depends on the reagent and manufacture used.25,26 There is also substantial geographic variation in the prevalence of sensitisation to aeroallergens, including fungi.27 In vitro measurement of specific IgE antibodies can be useful in patients who cannot undergo SPT. Smits et al.26 found that only 43% of patients reacted to both SPT and specific serum IgE tests when tested for common aeroallergens and foods. They recommended the use of both tests to gain a definitive diagnosis, as not all sensitivities will be identified through the use of one alone. These findings were similar to previous comparison results between STP and RAST.28,29 This was an expected finding as the reagents were from different manufactures which used different processing methods. Although, the CAP system represents progression from the previous RAST systems, the extracts that are used to produce the CAP reagents are not available for the production of SPT reagents. In this study, six patients showed negative responses to SPT, but positive responses to serum specific IgE for Af by CAP system. Also, seven patients showed negative responses to serum specific IgE by CAP, while showing positive responses to SPT. If we had used SPT alone, we would have missed six cases of Aspergillus sensitisation and would have missed seven cases if we had used specific serum IgE tests alone. These findings suggested that both SPT and serum specific IgE tests should be performed simultaneously in order to more accurately evaluate the sensitisation status of Af in asthma patients.
An elevated level of Af-specific IgE antibodies, as measured by fluorescent EIA, has been considered the hallmark of ABPA detection.18 A cut-off value of IgE/IgG for Af of more than twice of the value from the pooled serum samples from asthma patients sensitised with Af can greatly improve the diagnosis of ABPA from other conditions.30 On a practical note, it is impossible to obtain specific reference values of serum IgE/IgG for Af according to various laboratory methods which allow for easy ABPA diagnosis under clinical settings. In our investigation, we incorporated the reference value of more than 0.35kU/L by CAP system into the measurement of specific IgE to Af according to a previous report.18 However, Agarwal et al.18 did not incorporate a reference value twice that of the pooled serum sample from asthma patients sensitised with Af.
With respect to the IgG measurement by CAP system, 35.0mgA/L was incorporated as a reference value according to the previous report.19 Hoeyveld et al.19 reported that reference values above 35.0 mgA/L for Af by CAP system had a sensitivity of 90% in detecting ABPA patients. Additionally, agreement between the precipitation technique and CAP system has been previously determined to be 86% for Af. By EIA, a reference value of IgG specific for Af above 12U/L was recommended by the manufacturer's protocol used for this study. Nine patients showed negative responses to IgG for Af by CAP system while demonstrating positive responses to EIA. This result may suggest that enzyme-linked immunosorbent assay (ELISA) is even more sensitive and reliable than immunodiffusion,12,13,30 the suboptimal specificity of which can lead to false-positive results, identifying clinically irrelevant IgG responses.
A reference value greater than 417kU/L or 1000ng/mL of total IgE by CAP system was used to define ABPA in this study as in previous studies.8,31 Other investigations also incorporated 1000kU/L as a cut-off point.18,21 Overall, we believe these two reference values have advantages as well as disadvantages. The former one carries a greater risk of over-diagnosing ABPA, but the latter carries a greater risk of missing the diagnosis. Because the total IgE concentration can be influenced by administration of systemic corticosteroids, we assume that the former is more appropriate for the diagnosis of ABPA. Overall, a follow up evaluation of total IgE for a specified period is a critical step to reduce the risk of missing diagnosis of ABPA.
Recently, Agarwal et al.32 reported that the immunological activity and outcomes of ABPA could be predicted on high resolution CT such as the chest findings of high-attenuation mucus (HAM), a marker of inflammatory activity. They postulated that the presence of HAM defines a subgroup of patients with more severe inflammation and is also useful for the prediction of relapse. Further studies are required to evaluate the usefulness of HAM in the classification of ABPA.
There are several limitations within our study. First the study was of a limited population of asthmatics who had eosinophilia with more than 1000cells/μL determined upon peripheral blood examination, and thus, further studies of larger populations of asthma patients are required in order to generalise these findings. Second, we did not perform an intradermal skin test with an extract of Af, and so, we could not evaluate the role of an intradermal test in the diagnosis of ABPA within a clinical setting. Third, we did not present radiological findings of the study patients. Fourth, there is potential for the exclusion of subjects with severe ABPA requiring systemic steroids for symptom control. Finally, we could not present cut-off values of IgE/IgG for Af from the pooled serum samples from asthma patients sensitised with Af, as the number of study patients in our study was too small. In conclusion, among patients who have negative responses to SPT for Af, the measurement of serum specific IgE antibody by CAP system may be helpful in evaluating the sensitisation status for Af. Since the clinical phenotype is changeable or variable in ABPA, more delicate laboratory reference values should be established in order to more accurately detect ABPA within asthma patients.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this investigation.
Patients’ data protectionThe authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.
Conflict of interestThe authors have no conflict of interest to declare.
This work was supported by Dong-A University research fund.