Systemic mastocytosis (SM) is characterised by the accumulation of neoplastic mast cells in bone marrow and other organs. In contrast with normal mast cells, in SM these cells usually express CD25.1 Urticaria pigmentosa (UP) constitutes the most common form of cutaneous affectation caused by SM. Urticaria pigmentosa, which is characterised by pruritus and reddish or violet skin lesions usually caused by the release of histamine and other inflammatory mediators, is generally unleashed after exposure to physical stimuli, changes in temperature, anxiety, or medication. Neoplastic mast cells may also infiltrate the gastrointestinal (GI) tract; in fact, GI symptoms in the form of colicky abdominal pain and diarrhoea are common in both SM and UP and may markedly alter patient quality of life. In this context, an increase in mast cells in GI biopsies has been proposed as a helpful criterion to establish the diagnosis of SM. Still, while an increase in mast cells has been reported in various inflammatory diseases, mast cell density has not been systematically evaluated in many GI disorders.
We have had the opportunity to study a female patient diagnosed with SM while also suffering from celiac disease (CD) in whom the establishment and maintenance of a gluten-free diet led to a good response in both diseases.
The subject was a 21-year-old female suffering from recurrent episodes of headache for the past five years and who had contracted infectious mononucleosis at the age of 19. In the family background, several first-degree maternal relatives had been diagnosed with CD, including the patient's mother, grandfather, three uncles, and four cousins. The patient had been presenting a persistent maculopapular rash on the neck and upper chest for the past three years. Armpit skin biopsies demonstrated multifocal and diffuse aggregates of tryptase(+) mast cells with ovoid nuclei in papillary dermis extending into the reticular dermis. CD117/c-kit immunostaining also resulted positive. Bone marrow aspirate resulted normal; subsequently, the c-kit D816V activating mutation was found on archived formalin-fixed paraffin-embedded skin tissue, which led to a diagnosis of SM in its childhood form of UP, including systemic manifestations.2
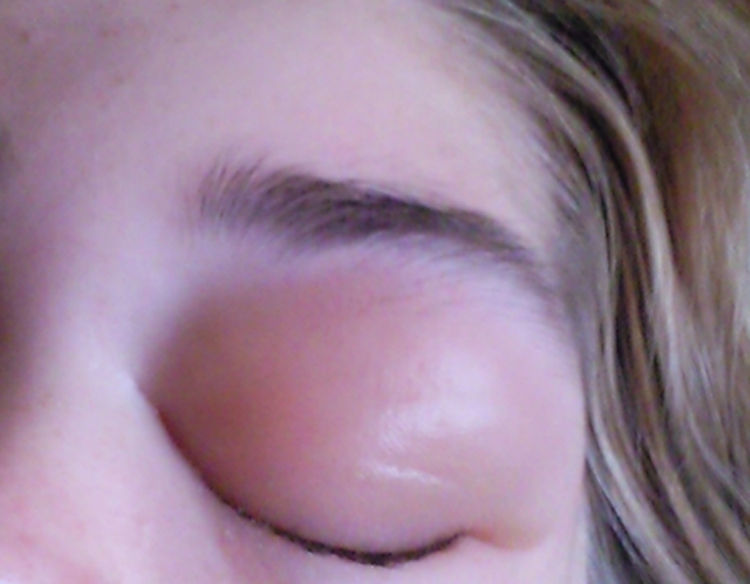
Physical exploration showed neck adenopathies and mild splenomegaly (with a bipolar diameter of 14cm). Every two or three months the patient had recurrent episodes of oedema in the left eyelid (Fig. 1) associated with increased tryptase serum levels around 30ng/mL, which resolved with intravenous steroid boluses. She also suffered pain in both legs and frequent episodes of generalised pruritus. Blood cell counts were normal, with the eosinophils count being below 200cells/μL. Liver function tests were normal as were total IgE serum levels (24kU/L). Grass-allergen specific IgE levels were therefore not determined.
Furthermore, she suffered from repeated episodes of abdominal pain and gastro-oesophageal reflux, along with sporadic diarrhoea. Because of this, she was referred to our Gastroenterology Department.
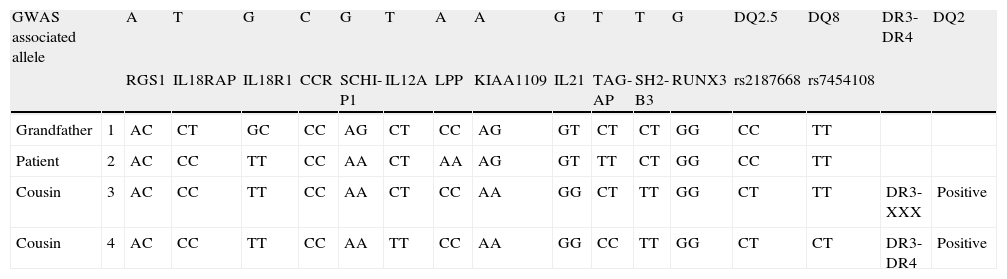
Analytical studies also showed decreased levels of cholesterol down to 120mg/dL (normal 150–250); decreased folic acid serum levels (2.2ng/mL, normal 4.6–18.7) and reduced levels of vitamin B12 (193ng/mL, normal 211–946) without anaemia or iron deficiency. Anti-tissue transglutaminase antibodies resulted negative as did the genetic markers HLA-II types for CD, both HLA-DQ2 and DQ8. Duodenal biopsies showed an increased intraepithelial lymphoid infiltration of 27%, compatible with lymphocytic enteritis or the Marsh I stage for CD. Gastric biopsies exhibited a dense CD25(+) mast cell infiltration while a Helicobacter pylori search resulted negative both in biopsies and in the urease breath test. We recommended a gluten-free diet (GFD), which the patient followed strictly. Several weeks later she reported a great clinical improvement, including the disappearance of headaches, pruritus, leg pain, and abdominal pain. Moreover, the patient presented only a single episode of left eyelid oedema in a six-month period. Serum levels of folic acid and vitamin B12 also normalised. In order to confirm the suspected diagnosis of CD despite the absence of susceptible HLA haplotypes, the genetic analysis was expanded to include genome-wide association studies (GWAS) comparing our patient with her maternal grandfather and two cousins, all of whom had confirmed diagnoses of CD (Table 1). Our patient's pattern resulted similar to that of her cousins and very close to that of her grandfather, thereby allowing us to establish a diagnosis of CD.
Genetic analysis of genome-wide association studies (GWAS), comparing the presented patient with her maternal grandfather and two cousins, all sufferers of CD.
| GWAS associated allele | A | T | G | C | G | T | A | A | G | T | T | G | DQ2.5 | DQ8 | DR3-DR4 | DQ2 | |
| RGS1 | IL18RAP | IL18R1 | CCR | SCHI-P1 | IL12A | LPP | KIAA1109 | IL21 | TAG-AP | SH2-B3 | RUNX3 | rs2187668 | rs7454108 | ||||
| Grandfather | 1 | AC | CT | GC | CC | AG | CT | CC | AG | GT | CT | CT | GG | CC | TT | ||
| Patient | 2 | AC | CC | TT | CC | AA | CT | AA | AG | GT | TT | CT | GG | CC | TT | ||
| Cousin | 3 | AC | CC | TT | CC | AA | CT | CC | AA | GG | CT | TT | GG | CT | TT | DR3-XXX | Positive |
| Cousin | 4 | AC | CC | TT | CC | AA | TT | CC | AA | GG | CC | TT | GG | CT | CT | DR3-DR4 | Positive |
RGS1: regulator of G-protein signalling 1; IL18RAP: interleukin 18 receptor accessory protein; IL18R1: interleukin-18 receptor 1; CCR: CCR gene complex; SCHI-P1: schwannomin interacting protein 1; IL12A: interleukin-12 subunit alpha; LPP: lipoma-preferred partner; IL21: interleukin 21; TAG-AP: T-cell activation RhoGTPase activating protein; SH2-B3: SH2B adaptor protein 3; RUNX3: runt-related transcription factor 3.
Food allergies are immune-mediated responses to food proteins comprising three types of mechanisms: (a) IgE pure-mediated disorders, including urticaria, angio-oedema, asthma, and gastrointestinal anaphylaxis, among others; (b) mixed IgE-cell-mediated disorders, including atopic dermatitis and eosinophilic gastrointestinal disorders; and (c) cell-mediated food allergies, which include dietary protein-induced procto or enterocolitis, food-induced pulmonary haemosiderosis, and CD.3 This last condition is an autoimmune-mediated enteropathy triggered and maintained by permanent intolerance to gluten in the diet which affects genetically susceptible individuals. Far from being considered an exclusively digestive disease, CD is widely recognised today as a systemic disorder, in which endocrine and metabolic alterations have long since been identified.
CD in adults usually manifests itself through atypical symptoms and often presents lymphocytic enteritis in duodenal biopsies. It is commonly associated with hypersensitivity and allergic manifestations in both the upper respiratory tract and skin.4–6 CD is often associated with other autoimmune diseases, acting through different pathogenic mechanisms such as chronic idiopathic urticaria, cold urticaria, and eosinophilic oesophagitis. Although the prevalence of chronic urticaria and cold urticaria in CD has yet to be determined, an increase in atopic immunological disorders has been reported within the context of gluten enteropathy; in fact, a poor course of cold urticaria in the absence of any other underlying condition should lead to suspicion of CD.7,8
Our patient exhibited a slight malabsorption pattern, with a decrease in cholesterol, folic acid, and vitamin B12 serum levels, all of which improved after following a GFD. Her clinical response was likewise favourable, with a decrease in headache, muscle aches, fatigue, episodes of eyelid oedema, and pruritus. Several authors have also shown that a mild enteropathy is symptomatic in some patients. Additionally, those patients showing Marsh I–II histological lesions usually have a reduction in gut inflammation when following a GFD, together with marked alleviation not only in gastrointestinal symptoms, but also in associated extra-intestinal diseases.4,5
Despite suffering from CD, our patient showed no HLA-DQ2 or DQ8 alleles. This phenomenon has been described in 6% of the European celiac population.9 In the last few years, GWAS has been performed on large numbers of CD patients, relatives, and match controls have revealed evidence of additional non-HLA loci of CD susceptibility, most of which are related to T-cell regulation and inflammation.9,10 In the light of this finding, and because CD affects 2% of the European and US populations at some stage in life, it would be advisable to investigate the presence of lymphocytic enteritis in patients with SM and UP, recommending a GFD in the case of a positive result, especially given the beneficial effects observed with no side effects. Such recommendations could markedly improve patient quality of life and increase the effectiveness of currently employed treatments.
Conflict of interestThe authors have no conflict of interests to declare.
Ethical disclosuresProtection of human and animal subjects. The authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
Confidentiality of data. The authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consent. The authors declare that no patient data appears in this article.