Stevens–Johnson syndrome (SJS) and Lyell's syndrome or toxic epidermal necrolysis (TEN) is the most serious and potentially fatal mucocutaneous reactions in paediatric patients. Both diseases are currently considered hypersensitivity variants of the same disease. The condition is referred to as SJS in patients with typical skin lesions covering less than 10% of the body surface, whereas the cutaneous detachment of more than 30% of the body surface leads to TEN. The intermediate percentages (between 10 and 30%) are defined as an SJS-TEN overlap.1
The incidence of SJS and TEN is low and is estimated to be between 1.5 and 2.0 cases per million people per year in the general population. Although these reactions are infrequent, they can cause grave sequelae and even lead to death.1
Various aetiological factors have been implicated as precipitating the hypersensitivity reactions that occur mainly in SJS and TEN, particularly infectious agents, systemic diseases, neoplasms, radiation therapy, vaccines, and multiple drugs, identifying these agents as causative in 65% of cases. More than 200 drugs have been included as possible causes in the development of these diseases. The most common of these drugs are anticonvulsants, antibiotics (such as sulphonamides and penicillins), allopurinol, and non-steroidal anti-inflammatory drugs (NSAIDs).1,2
Although the pathogenesis of both entities (SJS-TEN) is not yet fully understood, it is recognised that the final mechanism is the apoptosis of keratinocytes. This phenomenon is due to the Fas/FasL interaction, cytotoxic T cells, TNF-alpha, and nitric oxide synthase.2
There is no specific treatment for SJS and TEN. The first step is to suspend the use of the drug suspected to be the cause. Concurrently, supportive care should be administered, and any complications should be treated. The state of hydration and nourishment of the patient is essential for the disease management.1,2 Considering the immunological basis of SJS and TEN, the use of intravenous immunoglobulin (IVIG) as a treatment in paediatric patients has been proposed, with certain studies reporting successful results.2 In this work, two SJS-TEN patients, in whom IVIG was successfully used, are presented, and the existing literature is reviewed.
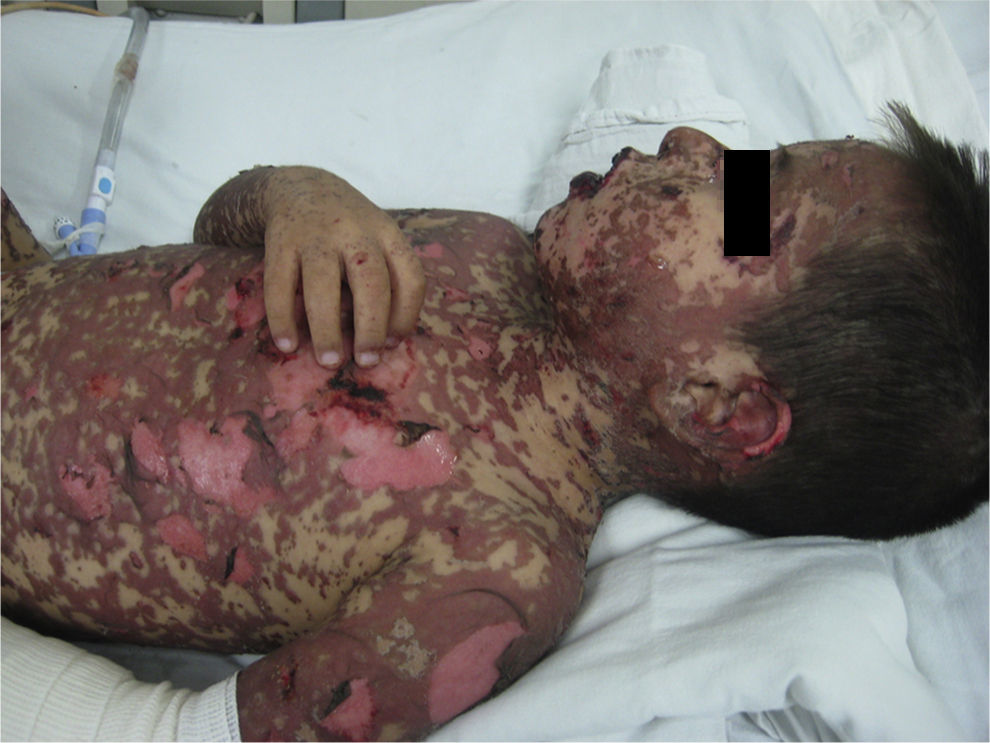
The first case was a four-year-old male patient diagnosed with Doose syndrome, who was prescribed Lamotrigine (Lamictal) for generalised atonic and tonic myoclonic seizures that were difficult to stabilise. Three weeks later, he was admitted, suffering from eight days of disease development characterised by fever up to 40°C, headache, asthenia, adynamia, and pruritus, as well as the onset (on the 5th day) of a generalised dermal eruption on the face, with progression to the trunk, limbs, and genitals. On admission, the patient presented with widespread dermatosis manifested by papuloerythematous injuries and blisters that were mainly on the face, neck, trunk, limbs, and genitals; with necrotising lesions and loss of continuity characterised by patches; and with a positive Nikolsky sign and blisters that converged into bullae of serous content, involving only the palms and soles. The affected body surface area was calculated to be 70%, which by definition was considered TEN (Fig. 1).
Case 1. The patient presented with widespread dermatosis manifested by papuloerythematous injuries and blisters that were mainly on the face, neck, trunk, limbs, and genitals; with necrotising lesions and loss of continuity characterised by patches; and with a positive Nikolsky sign and blisters that converged into bullae of serous content, involving only the palms and soles.
Bilateral conjunctival erythema was observed, in addition to purulent discharge in the oral cavity, with abrasive damage, fibrin plates, and halitosis, as well as erosive zones on the hard palate, gums, and tongue. Management was initiated using total parenteral nutrition, intravenous solution, electrolyte requirements, 40mg/kg/day clindamycin, and 900mg/kg/day IVIG (five doses).
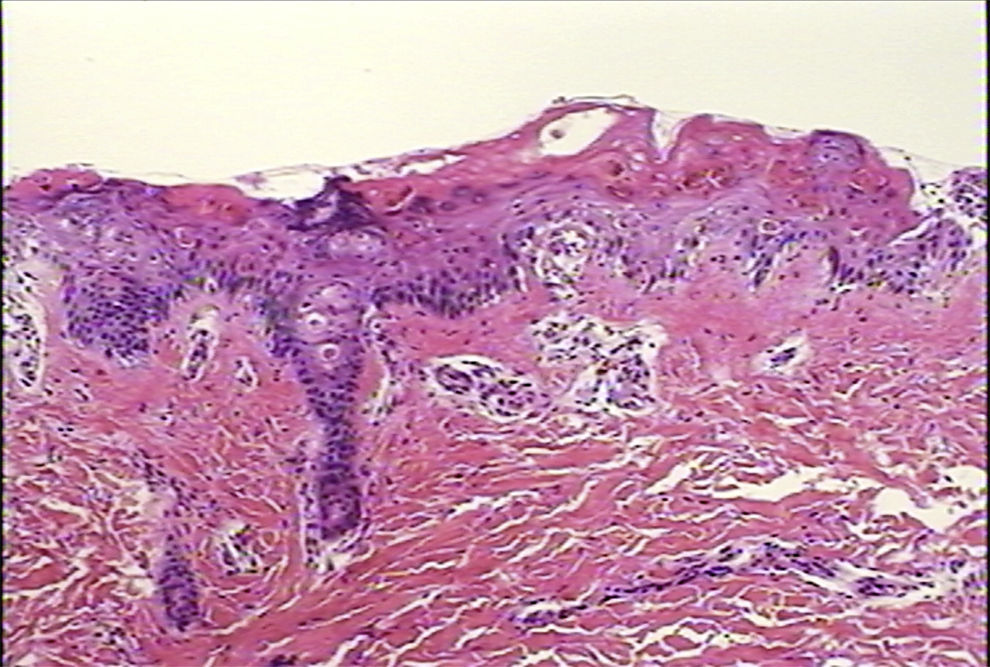
Ten days after beginning the IVIG treatment, the patient was reported to be afebrile, with marked improvement and 80% re-epithelialisation of the papuloerythematous and ampullary lesions. The skin biopsy is described in the figure legend (Fig. 2). The patient remained hospitalised for 14 days and was subsequently discharged without complications.
Case 1. Skin biopsy displaying the altered epidermis. Degenerative vacuolar changes are observed in the dermoepidermal junction, accompanied by alterations in the stratified maturation of the acanthocytes. The inflammatory exudate is extremely scarce and is limited to the papillary dermis. Haematoxylin and eosin (H–E) stain, 10×.
The second case was a ten-year-old female patient with a history of convulsive crises, who required hospital admission with a diagnosis of benign rolandic epilepsy and was released after being prescribed 10mg/kg/day oxcarbazepine. She was readmitted 22 days later, presenting erythroderma on her face, neck, and anterior thorax, as well as angio-oedema of her eyelids, lips, and neck. Twenty-four hours later, the patient also exhibited papuloerythematous, bullous eye lesions that extended by peripheral expansion into the thorax with a few vesicles and bullae with a positive Nikolsky sign. The lesions were accompanied by hyperaemia and bilateral conjunctival secretions, a seropurulent secretion from the right ear, and vesicles in the oral mucosa. SJS was diagnosed, and the patient was admitted to the Paediatric Intensive Care Unit (PICU), with an indication to suspend the oxcarbazepine.
It was decided to start management with 750mg/kg/day IVIG for (four doses), intravenous fluids up to 1800m2/day body surface, and 40mg/kg/day clindamycin. Eight days after beginning the treatment, the patient displayed marked improvement without a fever spike and with remission of her skin lesions, characterised by vesicles and bullae, and widespread desquamation.
Discussion: It has been reported that genetic susceptibility plays an important role in the pathogenesis of SJS and TEN; the HLA (human leucocyte antigen)-B*1502 is closely associated with carbamazepine-induced SJS in the Chinese population. Consequently, since 2007, the FDA has recommended determining this genotype in the population of Asian origin before starting treatment with carbamazepine.3 It is difficult to distinguish the early stage of symptoms in patients with SJS and TEN from other adverse drug-induced responses in the skin. A significant increase in the levels of soluble Fas ligand (FasLs) has been reported in subjects with SJS and TEN before the onset of mucosal lesions and epidermal sloughing. Therefore, it may be useful to determine the FasLs levels during the early stages of SJS and TEN.4
In a case series of 15 patients treated at the Children's Hospital of Mexico (1996–2005), Kuhn et al. reported that one-half of the cases were secondary to anticonvulsant treatment (two cases with phenytoin, three cases with carbamazepine, and two cases with lamotrigine).2 The results of the multinational study (EuroSCAR) involving 379 patients with adverse reactions validated as SJS or TEN confirmed that the drugs with greatest risk for causing these conditions include allopurinol (17.4%), carbamazepine (8.2%), cotrimoxazole (6.3%), phenobarbital (5.3%), and phenytoin (5.0%). The newly introduced drugs associated with increased risk for TEN and SJS included nevirapine (5.5% and relative risk [RR]>22) and lamotrigine (3.7% and RR>14).5 These studies linking lamotrigine as a relative risk factor for SJS and TEN are related to the presentation of our case of TEN secondary to this recently introduced anticonvulsant. In the second case of this review article, a patient with SJS induced by oxcarbazepine was presented. According to the reviewed literature, unlike carbamazepine, oxcarbazepine has rarely been reported to be associated with SJS and TEN. Lung-Chang et al. reported a case of a nine-year-old child with HLA-B*1518 who had oxcarbazepine-induced SJS.6
Corticosteroids are used to manage TEN because of their effects, including (but not limited to) inhibition of transcription factor NF-kB, which is involved in regulating the production of numerous pro-inflammatory cytokines, such as IL 1, IL 6, IL 8, IL 11, IL 12, IL 15, and IL 16, as well as, of particular relevance, IFN-gamma and TNF.7
IVIG acts at the level of the Fas receptor, thereby blocking its binding to FasL and preventing apoptosis of keratinocytes in SJS and TEN. In addition, IVIG also has effects that support its use in these conditions because IVIG decreases the infectious complications, reduces fluid loss through the denuded skin, and has immunomodulatory effects. The immunomodulatory activity includes the following: (1) actions on phagocyte receptors, with transient blocking within phagocytic cells and on FcRn (principal antibody ligand IgG) receptors; (2) actions in the complement system, with inhibition of the binding of C1, C3b, and C4b upon saturating the CR1 (ligand-binding specificity C3b and C4b) and CR3 (ligand-binding specificity iC3b, ICAM-1) receptors of phagocytes; (3) blockage of the active sites on autoantibodies, anti-IL1, IFN-gamma, and TGF-beta; and (4) protective effects against TNF-alpha damage.8
IVIG has been used successfully to treat paediatric patients with SJS and TEN. Metry et al. have reported the use of IVIG in seven cases and reviewed the literature.8 On the basis of their results, the authors recommended using high doses of IVIG (between 0.5 and 1.0g/kg/day) administered between three and four days as the most effective method to obtain a rapid response, particularly when this treatment is initiated early.
Various clinical practice guidelines (CPGs), such as those from the University of Florida, recommend administering IVIG to patients with SJS and TEN. The Clinical Guidelines for Immunoglobulin Use proposed by the Group of Experts of the Department of Health of Great Britain9 recommend IVIG use in SJS and TEN when other treatments are contraindicated or when the risk of mortality exists (grade B recommendation, IIa level of evidence).
Based on the currently available evidence, it is necessary to conduct controlled prospective and double-blind studies (which are difficult to perform due to the low incidence of SJS and TEN) to reaffirm the indication of IVIG treatment in these conditions.10
Ethical disclosuresConfidentiality of dataThe authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.
Protection of human subjects and animals in researchThe authors declare that no experiments were performed on humans or animals for this investigation.