To determine the possible impact of recurrent wheeze on immunisation status in the first three years of life.
MethodA cross-sectional case control study of 288 children less than three years of age with a history of recurrent wheezing, hospitalised at a single centre for wheeze; and a control group of 190 children with no prior history of wheezing .Vaccination charts of all children were analysed according to the National Immunisation Schedule. Additionally, some associated data were collected through a questionnaire to the parents.
ResultsChildren with recurrent wheezing during the first three years of life were less likely to be vaccinated against BCG (Bacillus-Calmette-Guerin), hepatitis B, Hib (Haemophilus influenza type B), and MMR (Measles, Mumps, Rubella) (p < 0.001). A significant inverse association was also found between the number of wheezy episodes and the number of vaccine doses received. The odds ratio of incomplete vaccination in children with recurrent wheeze was 10.6 (95% CI, 2.96 to 38.1).
ConclusionChildren under three years of age with recurrent wheeze run a high risk of incomplete immunisation. Efforts should be therefore made to insure that such children receive adequate vaccination.
Wheezing is very common in infants; 20% of them suffer from wheezing in early childhood.1–3 Although not all children who wheeze can be considered asthmatic, at least some of them are at increased risk of developing asthma in the later years of childhood.4 Vaccination is an important strategy to prevent certain viral and bacterial infections that threaten the health and life of children, including chronically sick children, like paediatric wheezers. Asthma is characterised by Th2-dependent inflammation. Atopic subjects have typically Th-2 polarisation and reduced Th-1 response,5 which may render allergic children more susceptible to infections than non-allergic subjects.6 Transient early wheezing is related to common viral infections and tends to disappear by the age of three. The primary risk factor is reduced pulmonary function since birth.7 Non-atopic wheezing is related to acute viral infections, especially respiratory syncytial virus and occurs frequently with a seasonal pattern. It is a transient condition and tends to disappear during childhood.8,9 Atopic wheezing (asthma) is a chronic inflammatory process of the airways and is the result of the interaction between genetic factors (familial history of atopy), environmental factors (exposure to allergens and early allergy sensitisation), and non-specific adjuvant factors, such as tobacco smoke, air pollution and infections.10,11 On the other hand, risk factors or determinants for incomplete vaccination status in childhood are largely unknown and may show great differences based on child status.12–14 There is no contraindication for receiving a specific vaccine in children suffering from wheezing or high-risk children of atopic parents. The primary objective of this study was to assess the vaccination coverage and the risk factors associated with incomplete vaccination in children suffering from recurring wheeze.
Patients and methodsThis study was conducted at the Vakif Gureba Education and Research Hospital, Istanbul, Turkey, a tertiary teaching hospital, and was approved by the Institutional Committee for Ethics in Research. Two hundred and eighty-eight children, under three years of age who were hospitalised between January 10, 2008 and September 30, 2009 for recurrent wheeze were enrolled. All had a history of recurrent wheezing, defined as at least four documented wheezing episodes in the past. Wheezing was defined as an acute respiratory illness with tachypnoea, prolonged expiration, and wheezing on auscultation of the chest. One hundred and ninety infants of the same age, who were hospitalised for other diseases (e.g., urinary tract infections, anaemia, inguinal hernia) during the same period, with no history of wheezing, atopic disease or chronic illness, served as controls. After giving written informed consent, parents or legal guardians of the children were interviewed and then the vaccination charts of the children evaluated. Additionally, a detailed questionnaire was administered, which included some sociodemographic and disease associated characteristics (parental education; family income; number of siblings; vaccination status- completed, not completed, or never given; total number of wheezing attacks that required emergency admission during the last six months; place of residence; total breast-feeding time; antibiotics administered during the first year of infancy; total number of antibiotic prescriptions during the last six months; and the frequency of prophylactic or nebulised corticosteroid inhalations).Only charts that had been officially filled out with information about the vaccines were considered. Children's vaccination status was considered complete if they had the vaccination according to the National Vaccination Schedule (NVS), (Table 1).15,16 The recommended NVS includes one dose of Bacillus Calmette-Guérin (BCG), three doses of hepatitis B, three doses of Oral Polio Virus (OPV) and Diphtheria Pertussis Tetanus (DPT), one dose of Measles Mumps Rubella (MMR) and three doses of Haemophilus influenza type b (Hib). The vaccination status was considered incomplete if the children missed out on any of these vaccines. Additionally, at the beginning of 2009, Special Immunobiological Agents Programme (SIBAP) was taken up only for immunocompromised or other high-risk children. This does not include children with asthma. SIBAP includes pneumococcal, varicella vaccines and yearly influenza vaccine. Vaccines of NVS and SIBAP were government-sponsored and were freely offered to the patients. As no updated information on influenza vaccine was available for any of the patients, this vaccine was not included in the evaluation. Besides, this vaccine was a new addition to the SIBAP.
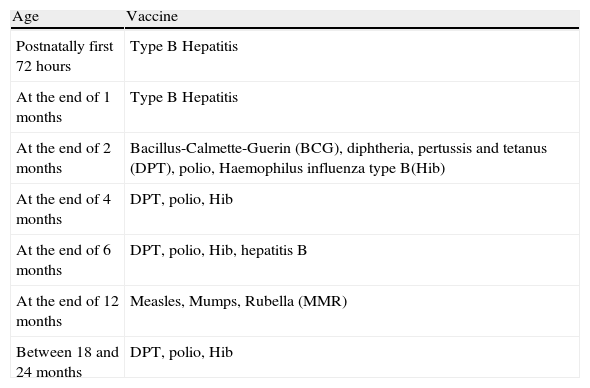
Turkey National Vaccination Schedule in 2008.15,16
| Age | Vaccine |
| Postnatally first 72 hours | Type B Hepatitis |
| At the end of 1 months | Type B Hepatitis |
| At the end of 2 months | Bacillus-Calmette-Guerin (BCG), diphtheria, pertussis and tetanus (DPT), polio, Haemophilus influenza type B(Hib) |
| At the end of 4 months | DPT, polio, Hib |
| At the end of 6 months | DPT, polio, Hib, hepatitis B |
| At the end of 12 months | Measles, Mumps, Rubella (MMR) |
| Between 18 and 24 months | DPT, polio, Hib |
The place of residence was classified as urban or rural, depending on whether it allows population breakdown by villages, towns and cities. This classification is based on population size. Cities and towns were considered urban and villages rural. Patients whose vaccination charts were not updated or incomplete were referred to the Immunisation Centre for vaccination before discharging them from the hospital.
Statistical analysisThe questionnaires were coded and the data entered using the EPI INFO 5.0 program and later converted to SPSS 11.5 (SPSS Inc., Chicago, USA) software. General characteristics were analysed by independent sample t-test or Mann-Whitney test depending on the data, and category-wise data by Pearson chi-square test. Association between factors and incomplete vaccination status was tested first by chi-square test. To investigate the relative importance of the variables in relation to dependent factors and in case there was any confounding between them, they were fitted together using a binary logistic regression model. Characteristics that were found significant through binary analyses were entered into a multivariate logistic regression model to control confounding factors and to determine which characteristics were independent of the vaccination status of the children with recurrent wheeze. A P-value of less than 0.05 or OR with a 95% confidence interval (CI) that did not include 1.00 was considered significant.
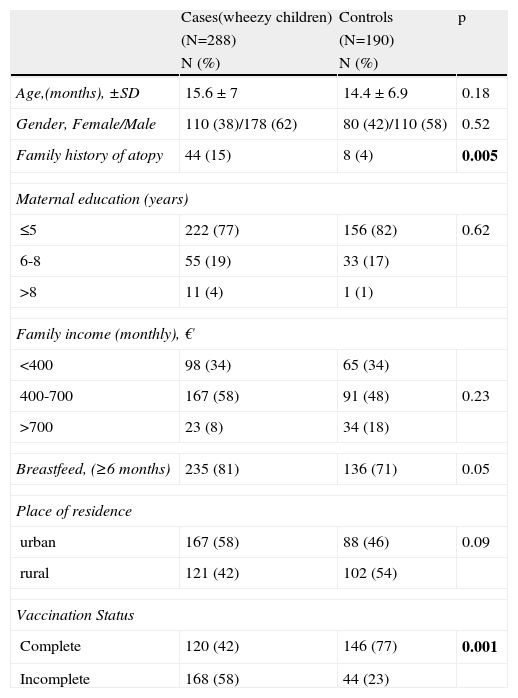
ResultsPopulation characteristicsA total of 478 subjects (288 cases and 190 controls) were included in this study. The mean age of the cases (15.6 months, SD=7) was similar to that of the controls (14.4 months, SD=6.9). The gender distribution was uniform, males constituting 62% of the cases and 58% of the controls. Several socio-demographic characteristics were found to be useful predictors of the vaccination status of children (Table 2). As there were no significant differences between the sociodemographic data of the two groups, except for two parameters (family history of atopy and the problem of wheeze), it was possible to obtain clear results in the comparison of the two groups. Family history of atopy was more common in the wheezing group than in the control group. The incidence of incomplete vaccination was statistically higher in the wheezy group than in the control group (58% and 23%, respectively, p <0.0001, Table 2).
Comparison of some sociodemographic characteristics of Cases and Controls.
| Cases(wheezy children) | Controls | p | |
| (N=288) | (N=190) | ||
| N (%) | N (%) | ||
| Age,(months), ±SD | 15.6 ± 7 | 14.4 ± 6.9 | 0.18 |
| Gender, Female/Male | 110 (38)/178 (62) | 80 (42)/110 (58) | 0.52 |
| Family history of atopy | 44 (15) | 8 (4) | 0.005 |
| Maternal education (years) | |||
| ≤5 | 222 (77) | 156 (82) | 0.62 |
| 6-8 | 55 (19) | 33 (17) | |
| >8 | 11 (4) | 1 (1) | |
| Family income (monthly), € | |||
| <400 | 98 (34) | 65 (34) | |
| 400-700 | 167 (58) | 91 (48) | 0.23 |
| >700 | 23 (8) | 34 (18) | |
| Breastfeed, (≥6 months) | 235 (81) | 136 (71) | 0.05 |
| Place of residence | |||
| urban | 167 (58) | 88 (46) | 0.09 |
| rural | 121 (42) | 102 (54) | |
| Vaccination Status | |||
| Complete | 120 (42) | 146 (77) | 0.001 |
| Incomplete | 168 (58) | 44 (23) | |
Incomplete vaccination was identified in 168/288 (58%) children with persistent wheeze; incompleteness in terms of BCG was noted in 104 (62%) children; in terms of Hib in 27 (16%) children; in terms of MMR in 20 (12%) children; and in terms of Hepatitis B in 17 (11%) children. In the control group, incomplete vaccination was identified in 44/190 (23%) children without persistent wheeze, and the corresponding figures are 27 (62%) for BCG, 10 (23%) for Hib, four (9%) for MMR, and three (7%) for Hepatitis B. Among the children with persistent wheeze, 46 (28%) children missed more than one vaccine, and among those of the control group 13 (29%) children.
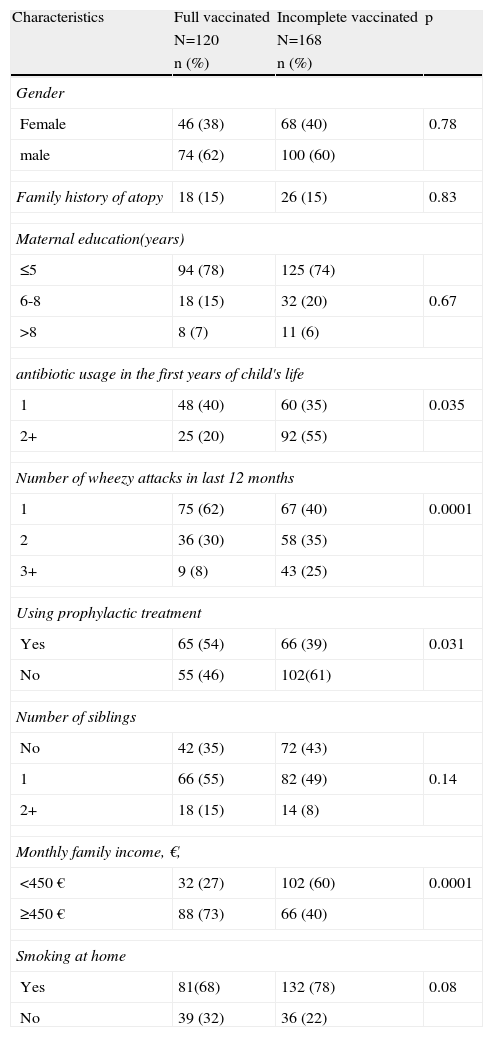
Bivariate analysis revealed significant statistical association between the incidence of incomplete vaccination and antibiotic usage during the first years of infancy, number of wheezy attacks during the last 12 months, family income, and avoidance of prophylactic treatment = asthma controller medication. Incidence of incomplete vaccination showed no association with the gender of child, mother's education level, family history of atopy, smoking by other household members at home, and more siblings than one (Table 3).
Relation of some characteristic with vaccination status of wheezy children (N=288).
| Characteristics | Full vaccinated | Incomplete vaccinated | p |
| N=120 | N=168 | ||
| n (%) | n (%) | ||
| Gender | |||
| Female | 46 (38) | 68 (40) | 0.78 |
| male | 74 (62) | 100 (60) | |
| Family history of atopy | 18 (15) | 26 (15) | 0.83 |
| Maternal education(years) | |||
| ≤5 | 94 (78) | 125 (74) | |
| 6-8 | 18 (15) | 32 (20) | 0.67 |
| >8 | 8 (7) | 11 (6) | |
| antibiotic usage in the first years of child's life | |||
| 1 | 48 (40) | 60 (35) | 0.035 |
| 2+ | 25 (20) | 92 (55) | |
| Number of wheezy attacks in last 12 months | |||
| 1 | 75 (62) | 67 (40) | 0.0001 |
| 2 | 36 (30) | 58 (35) | |
| 3+ | 9 (8) | 43 (25) | |
| Using prophylactic treatment | |||
| Yes | 65 (54) | 66 (39) | 0.031 |
| No | 55 (46) | 102(61) | |
| Number of siblings | |||
| No | 42 (35) | 72 (43) | |
| 1 | 66 (55) | 82 (49) | 0.14 |
| 2+ | 18 (15) | 14 (8) | |
| Monthly family income, €, | |||
| <450 € | 32 (27) | 102 (60) | 0.0001 |
| ≥450 € | 88 (73) | 66 (40) | |
| Smoking at home | |||
| Yes | 81(68) | 132 (78) | 0.08 |
| No | 39 (32) | 36 (22) | |
A multivariate logistic regression model was made with variables which were significantly associated with incomplete vaccination and these included the number of wheezy attacks, regular prophylactic treatment, antibiotic usage during the first years of infancy, and socio-economic status. Table 4 shows that the fully adjusted OR of 10.6 (95% CI=2.96-38.1) for number of wheezy attacks was still a statistically significant factor
Estimates of simultaneous effect of variables on the incomplete vaccination status of the recurrent wheezy children through logistic regression (N=168).
| OR | 95% CI | |
| number of wheezy attacks ≥3 | 10.6 | 2.96–38.1 |
| regular prophylactic treatment | 0.85 | 0.42–1.68 |
| antibiotic usage in the first years of child's life | 1.36 | 0.71–2.61 |
| socioeconomic status | 2.1 | 0.5–9.02 |
CI; confidence interval.
This study reveals that children with recurrent wheeze are inadequately immunised. Although some previous studies considered the role of chronic diseases of children in vaccination coverage,12,13 children with persistent wheeze were not either examined at all or only a limited number of them were included in those studies. None of the epidemiological studies of the control or comparison groups stress the risk of incomplete vaccination status in children with persistent wheeze.
According to the results of this study, no sociodemographic variable, except monthly family income, is related to children's incomplete vaccination status. This finding is consistent with that of some earlier studies.17,18 Contrary to this, Anand and Bärnighausen recently showed that the level of income does not contribute to improved immunisation coverage.19 Also, there was no evidence to support that child's gender had any relation to vaccine uptake or to defining incomplete vaccination status of the present study.20 In some societies where cultural discrimination against female children is prevalent, boys stand a greater chance of being vaccinated.21 Similarly, the age of mothers was not seen to be associated with the vaccination status. In some studies, both younger22 and older mothers23 were reported to be associated with incomplete vaccination. Association of low education level of mothers with low infant vaccination uptake was reported earlier,21–23 but the present study shows no such association. This is probably because very few mothers had more than college or school education. This finding is consistent with that of some earlier studies.24,25
Recurrent wheeze is most strongly associated with atopy.26,27 Factors associated with recurrent wheeze are mainly related to markers of atopic susceptibility and to exposure to infections.27,28 Thus, allergic children are more easily infected as allergic reactions are characterised by mucosal inflammation that predisposes the children to infections. Consequently, the allergic children tend to use more antibiotics than the non-allergic children.29 On the other hand, taking antibiotics for respiratory disease is due to the confounding effects of chest infection at an age where this may be difficult to separate from asthma-related wheeze.
The number of wheezy attacks was found to be the only one significant predictor of incomplete vaccination status of a child with recurrent wheeze; it does seem that greater number of wheezy attacks could have been the reason for incomplete vaccination status. The reasons for this may include recurrent wheezing attacks necessitating hospitalisation that may cause a delay in receiving age-appropriate vaccination, or medical staff or parents believing incorrectly that vaccination may aggravate wheezing illness.The limitations of this study are the small sample size, and the fact that the cases and controls were drawn from a single hospital. As the study covered only children from one hospital, it is possible that outside the hospital there could be many more children with more severe persistent wheeze who form part of this population, but were excluded from this study.
To improve the quality of life of children with persistent wheeze, paediatricians or health care providers responsible for patients’ management at the level of emergency clinic or hospital, should keep track of the vaccination status of these children. Children with persistent wheeze may need additional support and encouragement in getting complete immunisation. The vaccination charts should be regularly monitored and updated so as to prevent the incidence of immuno-preventable diseases in children with high risk, such as persistent wheeze.
Conflict of InterestThe authors declare that they have no conflict of interest.