Contrast-induced acute kidney injury is the third leading cause of intrahospital acute kidney disease, accounting for 11% of all cases. It is associated with a worse prognosis on the short- and long-term, as well as with extended hospital stay and increase in health-care costs. As the number of diagnostic and interventional angiographies and computerized tomography increases in clinical practice and higher doses of contrast media are administered to sicker and older patients, contrast-induced acute kidney injury is an increasing problem. Contrast-induced acute kidney injury is a unique form of acute kidney injury in that its risk factors are known and its timing predictable. This makes room for the implementation of prophylactic measures in patients at risk.
Several articles were searched in nephrology journals (“American Journal of Kidney Disease”, “Journal of the American Society of Nephrology”, “Clinical Journal of the American Society of Nephrology”, “Kidney International” and “Nephrology Dialysis Transplantation”) for a global view on contrast-induced acute kidney injury and prophylactic strategies. Subsequently, individual searches were made on MEDLINE® database for randomized controlled trials and meta-analyses on each prophylactic strategy encountered.
Several approaches to contrast-induced acute kidney injury prevention have been reported, of which vigorous hydration and the use of non-ionic contrast media are the most important. The administration of oral N-acetylcysteine is also a popular strategy in virtue of its favorable risk/benefit profile. Statins have also been reported as protective against contrast-induced acute kidney injury. The authors review the disease and studied prophylactic interventions, presenting a practical approach to the prevention of contrast-induced acute kidney injury.
A lesão renal aguda associada ao uso de contraste iodado é a 3ª causa mais frequente de lesão renal aguda intra-hospitalar, sendo responsável por 11% de todos os casos. A lesão renal aguda induzida pelo contraste associa-se a pior prognóstico a curto e longo prazo, bem como a maior duração do internamento hospitalar e maiores custos. À medida que o número de angiografias diagnósticas e terapêuticas e tomografias computorizadas aumenta na prática clínica e que maiores doses de contraste são administradas a doentes com idades mais avançadas e maior número de co-morbilidades, a lesão renal aguda induzida pelo contraste é um problema crescente. A lesão renal aguda induzida pelo contraste é uma forma única de lesão renal aguda na medida em que os factores de risco são conhecidos e é previsível no tempo. Assim, torna-se possível a implementação de medidas profilácticas nos doentes em risco.
Os autores pesquisaram artigos sobre lesão renal aguda induzida pelo contraste e estratégias profilácticas em diversas revistas nefrológicas (“American Journal of Kidney Disease”, “Journal of the American Society of Nephrology”, “Clinical Journal of the American Society of Nephrology”, “Kidney International” and “Nephrology Dialysis Transplantation”). Posteriormente, foram efectuadas pesquisas individuais na base de dados MEDLINE® de estudos randomizados controlados e metanálises para cada estratégia profiláctica encontrada.
Várias abordagens na prevenção da lesão renal aguda induzida pelo contraste têm sido descritas, das quais a hidratação vigorosa e o uso e contraste iodado não-iónico são as mais importantes. A administração de N-acetilcisteína oral é também uma estratégia popular em virtude do seu favorável perfil de risco/benefício. As estatinas têm vindo a ser descritas como protectoras contra a lesão renal aguda induzida pelo contraste. Os autores fazem uma revisão da patologia e das intervenções profilácticas testadas, propondo uma estratégia preventiva.
Contrast-induced acute kidney injury (CI-AKI) is a form of AKI that occurs after the exposure to intravascular iodinated contrast media.1,2 It is responsible for 11% of the cases of hospital acquired acute kidney injury and is the third commonest cause of acute tubular necrosis in patients admitted to hospital, after impaired renal perfusion and the use of nephrotoxic medication.3 Up to 10% may need temporary dialysis. Patients with severe kidney disease prior to contrast administration may progress to end-stage kidney disease. Its occurrence is associated with increased risk of complications and death on short-4,5 and long-term5–7 and leads to extended hospital stay4,5,8,9 as well as to increased health-care costs.5
As the number of diagnostic and interventional angiographies and CT examinations increases in clinical practice and higher doses of contrast media are administered to sicker and older patients, CI-AKI is an increasing problem.2 It remains one of the most clinically important complications of the use of iodinated contrast media.6,10
CI-AKI is unique is that its risk factors are known, it is universally iatrogenic and its timing is predictable. Also, in virtue of the elective nature of most of the radiologic procedures that require iodinated contrast administration, contrast nephropathy is probably one of the few preventable forms of AKI and a condition for which standardized preventive strategies would be feasible and effective.11
MethodsSeveral articles were searched in nephrology journals (“American Journal of Kidney Disease”, “Journal of the American Society of Nephrology”, “Clinical Journal of the American Society of Nephrology”, “Kidney International” and “Nephrology Dialysis Transplantation”) for a global view on contrast-induced acute kidney injury and prophylactic strategies. Subsequently, individual searches were made on MEDLINE® database for randomized controlled trials and meta-analyses on each prophylactic strategy encountered.
DefinitionCI-AKI is the acute deterioration of renal function after parenteral administration of radiocontrast media that cannot be attributed to other causes. The diagnosis of CI-AKI requires an absolute or relative increase in serum creatinine (SCr), temporally related to the parenteral administration of the contrast agent and exclusion of alternative explanations for renal impairment.1 The most common definition used in epidemiologic studies6 and clinical trials10 is an increase in SCr≥0.5mg/dL or ≥25% above baseline within 24 to 72h after contrast administration. Despite the recent definition of acute kidney injury by the Acute Kidney Injury Network group, that is an increase in SCr≥0.3mg/dL within 48h, most studies continue to use the former definition of CI-AKI.66,71,72
Epidemiology/risk factorsThe reported incidence of CI-AKI varies widely depending on the definition used, the presence of risk factors, especially chronic kidney disease and diabetes mellitus, and the amount and type of contrast media administered. Among patients with no risk factors for CI-AKI, namely chronic kidney disease, the risk is negligible.12 Among high-risk patients, the reported risk following percutaneous angiography is 10–30%.2,6,10,12,13
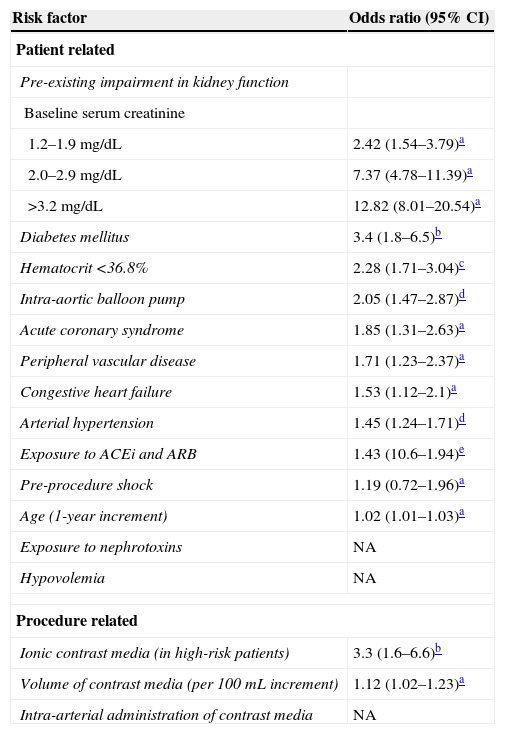
The most important risk factor for CI-AKI is pre-existing impairment of kidney function. The magnitude of the risk is directly associated with the severity of renal dysfunction6 and is potentiated by the association with diabetes mellitus and with other risk factors. Isolated diabetes mellitus, without concurrent kidney dysfunction, does not appear to be a significant risk factor, acting instead as a risk multiplier.1,6,12–14 Additional risk factors for CI-AKI, as listed in Table 1, include increasing age, arterial hypertension, peripheral vascular disease, congestive heart failure, acute coronary syndrome, pre-procedure shock and the use of intra-aortic balloon pump.6,15 A low baseline hematocrit was also identified as an independent predictor of CI-AKI.16 The use of drugs affecting kidney auto-regulation, namely angiotensin-converting-enzyme inhibitors (ACEi) or angiotensin receptor blockers (ARB), has also been associated with an increased risk of CI-AKI.17 As for other causes of acute kidney injury, hypovolemia and exposure to nephrotoxic drugs, including non-steroidal anti-inflammatory drugs, aminoglycosides and amphotericin B among others, increase the risk of CI-AKI. Kidney transplant patients are also at an increased risk of CI-AKI because of the high prevalence of diabetes, hypertension, graft dysfunction and the concurrent use of nephrotoxic drugs, namely calcineurin inhibitors.1
Risk factors for CI-AKI.
| Risk factor | Odds ratio (95% CI) |
|---|---|
| Patient related | |
| Pre-existing impairment in kidney function | |
| Baseline serum creatinine | |
| 1.2–1.9mg/dL | 2.42 (1.54–3.79)a |
| 2.0–2.9mg/dL | 7.37 (4.78–11.39)a |
| >3.2mg/dL | 12.82 (8.01–20.54)a |
| Diabetes mellitus | 3.4 (1.8–6.5)b |
| Hematocrit <36.8% | 2.28 (1.71–3.04)c |
| Intra-aortic balloon pump | 2.05 (1.47–2.87)d |
| Acute coronary syndrome | 1.85 (1.31–2.63)a |
| Peripheral vascular disease | 1.71 (1.23–2.37)a |
| Congestive heart failure | 1.53 (1.12–2.1)a |
| Arterial hypertension | 1.45 (1.24–1.71)d |
| Exposure to ACEi and ARB | 1.43 (10.6–1.94)e |
| Pre-procedure shock | 1.19 (0.72–1.96)a |
| Age (1-year increment) | 1.02 (1.01–1.03)a |
| Exposure to nephrotoxins | NA |
| Hypovolemia | NA |
| Procedure related | |
| Ionic contrast media (in high-risk patients) | 3.3 (1.6–6.6)b |
| Volume of contrast media (per 100mL increment) | 1.12 (1.02–1.23)a |
| Intra-arterial administration of contrast media | NA |
CI, confidence interval; ACEi, angiotensin-converting-enzyme inhibitors; ARB, angiotensin receptor blocker; NA, non-available data.
Rihal CS, Textor SC, Grill DE, et al. Incidence and prognostic importance of acute renal failure after percutaneous coronary intervention. Circulation. 2002;105(19):2259–64.
Rudnick MR, Goldfarb S, Wexler L, et al. Nephrotoxicity of ionic and nonionic contrast media in 1196 patients: a randomized trial. Iohexol Cooper Study Kidney Int. 1995;47(1):254–61.
Nikolsky E, Mehran R, Lasic Z, et al. Low hematocrit predicts contrast-induced nephropathy after percutaneous coronary interventions. Kidney Int. 2005 February;67(2):706–13.
The type of radiologic procedure also influences the risk of CI-AKI. Among all procedures that require contrast media administration, diagnostic and interventional coronary and peripheral angiography is associated with the highest risk of CI-AKI. This probably results from a high volume of contrast and from its intra-arterial administration which is associated with higher acute intrarenal concentration. Moreover, advanced vascular disease, which is usually associated with diabetes and hypertension, and hemodynamic instability are more frequent in patients undergoing coronary angiography.18
Clinical presentationThe clinical presentation of CI-AKI involves an asymptomatic increase in SCr occurring within 24–72h of intravascular iodinated contrast administration, peaking on the third to fifth days. In the majority of patients, the transient rise in SCr is the sole manifestation of CI-AKI. Nevertheless, a small proportion of patients can present with oliguria and other complications of AKI, namely hyperkalemia, metabolic acidosis and uremic syndrome.4,19 Once CI-AKI is established, renal function remains depressed for 1–3 weeks in most cases.20 About 6–10% of CI-AKI patients require renal replacement therapy in the acute phase.6,13,19 Spontaneous recovery of renal function is to be expected. Occasionally, renal failure is irreversible and thus requires long-term dialysis, especially in those patients with baseline severe kidney dysfunction.19
Differential diagnosisAs previously stated, the diagnosis of CI-AKI requires the exclusion of other causes of AKI, namely pre-renal azotemia or ischemic acute tubular necrosis, acute interstitial nephritis and renal atheroemboli.5 The history of additional insults such as sepsis, hypotension and exposure to nephrotoxic drugs suggests the possibility of the first two diagnoses. Renal atheroemboli should be suspected after angiography in the presence of digital ischemia, livedo reticularis, eosinophilia, hypocomplementemia, when the onset of AKI is delayed for several days after the procedure and in the absence of improvement of kidney function in patients with diffuse vascular disease subjected to intra-arterial contrast administration and interventional procedures.21
PathophysiologyThe pathophysiology of contrast-induced nephropathy is still a matter of debate. Among the mechanisms proposed are altered renal hemodynamics with intrarenal vasoconstriction contributing to medullary hypoxia and direct cytotoxicity of radiocontrast not only to tubular cells but also to endothelial cells. Some mediators of injury are possibly endothelin, reactive oxygen species and adenosine. It is also hypothesized that the high viscosity of iso-osmolar contrast media can aggravate medullary hypoxia not only by the impairment of medullary blood flow but also by increasing tubular fluid viscosity and the intratubular resistance to flow. Probably all these mechanisms act in concert to cause CI-AKI.11,20,22
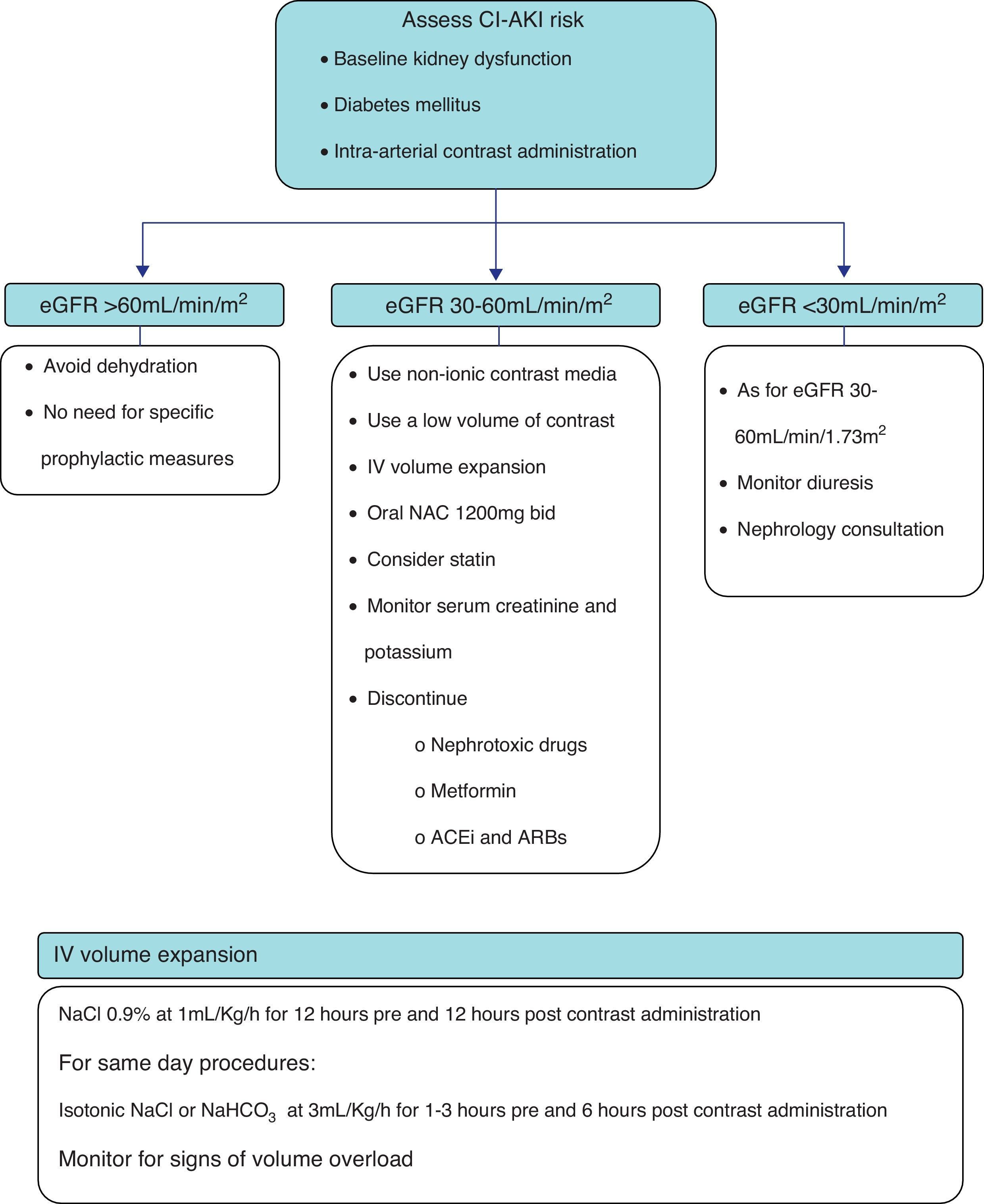
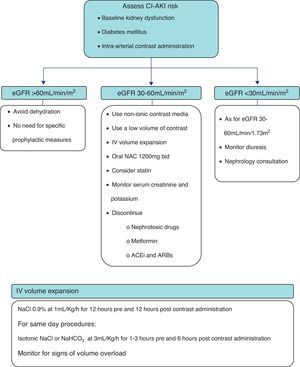
PreventionIn the absence of effective therapy and especially for iatrogenic diseases, prevention should always be the highest priority. Several approaches to CI-AKI prevention have been reported (Table 2), of which vigorous hydration and the use of non-ionic contrast media are the most important and widely accepted.20 The use of prophylactic oral N-acetylcysteine is also a popular strategy in high-risk patients. Many drugs and strategies have been tested in the prevention of CI-AKI, with disappointing results.23–29,38 Other strategies require further study.30,31
Tested strategies in the prevention of CI-AKI in high-risk patients.
| Strategies with proven benefit |
| Use of non-ionic contrast media |
| IV volume expansion with isotonic fluid |
| Strategies with potential benefit, requiring further study |
| Oral n-acetylcystein |
| Statins |
| Iloprost |
| Sodium citrate |
| Trimetazidine |
| Strategies that proved no benefit |
| Diuretics (furosemide, mannitol) |
| Theophylline |
| Fenoldopam |
| Non-selective endothelin receptor antagonist |
| Anaritide |
| Ascorbic acid |
Since the 1950s, the available contrast media have been based on triiodobenzene.20 They are commonly grouped according to their ionicity and osmolality. The first contrast media to be developed was ionic and high-osmolar. Latter were developed the low- and iso-osmolar contrast media which are non-ionic, except for ioxaglate.2,20 Since the introduction of non-ionic contrast media, the toxicity of these compounds has been mainly attributed to their osmolality, viscosity and chemotoxicity.2
Types of iodinated contrast agents.
| High-osmolar (1400–1800mosmol/kg)/Ionic |
| • Diatrizoate (Hypaque®, Gastrografin®, Urografin®) |
| Low-osmolar (500–850mosmol/kg) |
| Ionic• Ioxaglate (Hexabrix®)Non-ionic• Iohexol (Omnipaque®)• Iopamidol (Isovue®, Iopamiro®, Iopamiron®, Niopam®)• Iopromide (Ultravist®)• Ioversol (Optiray®) |
| Iso-osmolar (∼290mosmol/kg)/Non-ionic |
| • Iodixanol (Visipaque®) |
Today only low-osmolar contrast media (which still have considerably higher osmolality than plasma) and iso-osmolar contrast media are widespread because of the fewer adverse effects compared to high-osmolar contrast media,2 especially in high-risk patients with an elevated pre-procedural serum creatinine.2,12,20
EvidenceThe best data on the benefit of non-ionic contrast agents over high-osmolar, ionic ones comes from the iohexol cooperative study.12 This was a large multicenter double-blind randomized controlled trial (RCT) which demonstrated that the use of the non-ionic contrast agent iohexol is associated with significant less nephrotoxicity than the ionic contrast agent diatrizoate in patients with pre-existing renal failure alone or combined with diabetes mellitus undergoing elective coronary angiography. Patients with baseline SCr≥1.5mg/dL who received diatrizoate were 3.3 times more likely to develop nephrotoxicity compared to patients who received iohexol. In patients with normal baseline kidney function there was no evidence of reduced nephrotoxicity of the non-ionic agent iohexol compared to diatrizoate.
The NEPHRIC study10 reported a significantly lower incidence of CI-AKI with the use of the iodixanol iso-osmolar contrast media when compared to iohexol (low-osmolar) in patients with SCr 1.3–1.5mg/dL undergoing elective coronary or aorto-femural angiography. The better profile of this iso-osmolar contrast media was confirmed in the RECOVER trial,13 when compared to ioxaglate, an ionic, dimeric low-osmolar contrast agent. Nevertheless, subsequent trials comparing the use of iodixanol to other low-osmolar contrast agents (iopamidol14,32 and ioversol33) showed no significant difference in the incidence of CI-AKI in patients with chronic kidney disease undergoing elective coronariography with or without intervention. Low-osmolality contrast media seem to have different renal safety profiles. Iopamidol and ioversol appear to be as safe as the iso-osmolar contrast iodixanol. On the contrary, iohexol carries a higher risk of CI-AKI. This fact argues against the concept that osmolality is the primary determinant of renal toxicity, at least for low-osmolality contrast media.
ConclusionIn low-risk patients, mainly the ones with normal kidney function, the risk of CI-AKI remains low regardless of the contrast media used. In patients at high risk of CI-AKI, a non-ionic contrast, either low- or iso-osmolar, should be used in the lowest possible dose.
HydrationRationalThe precise mechanisms by which volume expansion protects against CI-AKI are unknown. It is speculated that volume expansion may act by counterbalancing the ischemic effect of radiocontrast on medullary cells. Dilution of the contrast media, particularly in the medullary tubular segments, may also reduce direct cellular damage. The increased tubular fluid viscosity, induced by the contrast agent, is reduced by intravascular volume expansion.11,20 The hypothesis that the alkalinization of the tubular fluid can reduce the formation of deleterious hydroxyl radicals and, hence, protect the renal medulla from oxidative stress injury has prompted the use of sodium bicarbonate in the prevention of CI-AKI.
EvidenceEarly data on the benefit of volume expansion in the prevention of CI-AKI come from observational, uncontrolled studies.11,34,35
A series of RCTs, mostly in high-risk patients undergoing elective or emergency coronariography, either diagnostic or interventional, have been undertaken to study the impact of intravenous fluid composition, rate and duration of administration on the prevention of CI-AKI. Most of these trials are small and underpowered.
Concerning the relative benefit of the use of isotonic (NaCl 0.9%) versus hypotonic sodium chloride (NaCl 0.45%) solutions, Muller et al.8 showed a smaller incidence of CI-AKI with the use of NaCl 0.9% (0.7% versus 2%, p=0.04) in a RCT enrolling 1620 patients. The benefit was more pronounced in diabetic patients and in those given >250mL of contrast. Despite the large number of patients enrolled in this study, most of the patients had normal baseline kidney function. In the small sub-group of patients with baseline SCr>1.6m/dL there was no difference in the incidence of CI-AKI in both study arms. Moreover, vascular complications, need for dialysis, length of hospital stay, and mortality rates were comparable between the two groups.
Knasuski et al.36 compared the use of a short intravenous hydration regimen with isotonic saline (20min pre- through 12h post-procedure) with a longer one using hypotonic saline in 5% dextrose (at least 12h pre- and 12h post-procedure) in chronic kidney disease patients. The incidence of CI-AKI in the shorter regimen was 10.8% compared to 0% in the control arm (p=0.136). The small number of patients (n=63) and lack of comparability between the two arms in the volume and composition of intravenous fluid administered limit the study's conclusion on the independent effect of timing and duration of therapy. Despite these limitations, these and the findings of Bader et al.37 in a small similar study suggest that the administration of intravenous fluid over a sustained period of time may be more protective than bolus fluid supplementation.
The results from some clinical trials comparing volume expansion with either isotonic NaHCO3 or isotonic saline have suggested the protective role of NaHCO3 in patients undergoing elective9,38 and emergent39 coronary angiography, while others have shown no benefit.40–42 Moreover, meta-analyses of trials on this matter have also yielded conflicting results.43–46 One of these meta-analyses45 found that bicarbonate therapy was most effective in coronary procedures, especially when emergent, and in patients with chronic kidney disease. Despite the heterogeneity of results relating to the prevention of CI-AKI, it is consensual across the studies that the need for renal replacement therapy43,44,46 and mortality43,46 does not significantly differ by fluid type. There is also no difference in the incidence of worsening heart failure or pulmonary edema with sodium bicarbonate in comparison to normal saline.43
With the increasing number of patients undergoing procedures that require iodinated contrast administration on an outpatient base, the effectiveness of oral hydration or salt loading in the prevention of CI-AKI has also been tested. A small RCT comparing the effect of unrestricted oral fluids to intravenous isotonic saline found a higher incidence of AKI in the former group.47 In a subsequent RCT enrolling 312 patients with stage 3 chronic kidney disease, oral saline hydration (1g/10kg of body weight of NaCl per os for 2 days before the procedure) was as efficient as intravenous saline hydration for the prevention of CI-AKI.48 Given the limited data, the safety and efficacy of oral hydration and salt loading remain uncertain in the prevention of CI-AKI.
ConclusionIn conclusion, it is generally accepted that volume expansion reduces the risk of CI-AKI and that isotonic intravenous fluids offer greater benefit than hypotonic fluid. Data suggest that isotonic NaHCO3 is not inferior to isotonic saline and may even provide additional protection against CI-AKI in chronic kidney disease patients undergoing coronary procedures. Moreover, its infusion over a relatively short period of time before and after contrast administration is safe and possibly effective. Additional studies are warranted before abbreviated bicarbonate intravenous fluid regimens become the standard of care.11 There remains a clear need for well-powered studies with large numbers of high-risk patients to answer the questions of composition, route of administration, rate and duration of volume expansion to prevent CI-AKI.11
The selection of fluid and rate of administration must take into account the patient's ability to tolerate the fluid load and the underlying risk for nephropathy. A possible approach is the administration of either isotonic fluid at a rate of 1mL/kg/h for 12h before and after contrast administration in high-risk hospitalized patients undergoing elective procedures. An alternative for patients undergoing urgent procedures or for whom sustained volume expansion is not possible is an abbreviated regimen of isotonic fluid at 3mL/kg/h 1h before and until 6h after the procedure.11
N-acetylcysteineRationalAs the increased oxidative stress seems to have a role in the pathogenesis of CI-AKI it seems reasonable to use anti-oxidants in its prevention. N-acetylcysteine (NAC) may prevent CI-AKI by stopping direct oxidative tissue damage and by improving renal hemodynamics.49
EvidenceSimilar to the effect of different regimens of intravenous fluid therapy, the relatively small RCTs on the effect of oral NAC in the prevention of CI-AKI have shown conflicting results. Some have shown a reduction of the risk of CI-AKI in high-risk patients49,50 while others have shown no benefit with the use of NAC.51–53 Most meta-analyses of RCTs on this matter have shown significant54–59 or at least a non-significant trend60 toward the benefit of the use of NAC in association with intravenous volume expansion in the prevention of CI-AKI. A finding common to all trials is the minimal toxicity of oral NAC. Recently, the largest RCT to date comparing the renal outcomes in patients undergoing coronary or peripheral angiography receiving oral NAC was published. Patients in the study arm received oral NAC 1200mg twice daily versus placebo, in association with intravenous volume expansion. This trial included 2308 patients and showed no benefit for NAC.61 However this study had several shortcomings: only about a third of the patients had chronic kidney disease (CKD) defined as a eGFR less than 60mL/min/1.73m2 and only about 16% had a SCr>1.5mg/dL. Also, the incidence of CI-AKI was low, even in patients with SCr>1.5mg/dL and over 20% of patients received high-osmolar contrast media.
Trials have also been undertaken on different NAC regimens. Brigori et al.62 undertook a RCT comparing standard dose (600mg twice daily) to high dose oral NAC (1200mg twice daily). They showed a smaller incidence of CI-AKI with the high dose regimen (3.5% versus 11%, p=0.038). Also, a meta-analysis on this matter, including mainly patients with CKD, showed an odds ratio of 0.46 for the occurrence of CI-AKI with the use of high dose NAC defined as a daily dose superior to 1200mg or a single pre-procedural dose above 600mg.63
Intravenous NAC has also been used in patients requiring emergent angiographic procedures in whom prophylaxis with the oral formulation in the day before contrast administration is not feasible. As with oral NAC the results were discordant.50,64 In the positive study,50 7% of the patients treated with intravenous NAC developed anaphylactoid reactions. Moreover, a recent meta-analysis has shown no significant benefit with the use of intravenous NAC.65
ConclusionIn conclusion, the use of high dose oral NAC in the prevention of CI-AKI, in association with intravenous volume expansion, is generally recommended given the possible benefit, the favorable side-effects profile, the low cost and ease of use. In contrast, the use of parenteral NAC is not recommended, due to not only to the lack of evidence but also to the reported adverse reactions.
StatinsRationalPleiotropic effects of statins include improved endothelial function, reduced inflammatory and immune-modulatory processes, improved oxidative stress and platelet adhesion. Statin treatment in patients with coronary/peripheral artery disease, as recommended by current guidelines, is associated with a lower incidence of major adverse cardiac events and lower mortality in the short- and long-term.66
EvidenceThe first evidence from the benefit of statins in the prevention of CI-AKI came from observational studies.67,68 RCTs on the effect of these drugs in the prevention of CI-AKI have again shown conflicting results. Earlier, relatively smaller studies on this matter, in patients with stable coronary heart disease, did not show a significant benefit.69,70 In the last few years, larger RCTs have shown the possible benefit of statins. Two studies have been undertaken in statin-naïve patients with acute coronary syndrome subjected to early percutaneous coronary intervention.71,72 These studies have shown the benefit of the pre-procedure administration of high doses of statins in association with intravenous hydration when compared to placebo and intravenous hydration (CI-AKI incidence 5% versus 13.2%, p=0.04671 and 6.7% versus 15.1%, odds ratio 0.38, 95% CI 0.2–0.71, p=0.00372). In one study,71 patients received 80mg of atorvastatin 12h before plus 40mg immediately before the procedure. In the other study, 40mg of rosuvastatin was administered at admission time.72 The 30-day incidence of adverse cardiovascular and renal events, namely need for dialysis and persistent renal damage, was significantly lower in the statin group.72 Patients with SCr>3mg/dL were excluded from both studies.
The largest RCT to date enrolled 2998 patients with diabetes mellitus and chronic kidney disease (creatinine clearance between 30 and 89mL/min/1.73m2) undergoing coronary or peripheral angiography and used standard doses of rosuvastatin.73 Patients in the study arm received a daily dose of 10mg of rosuvastatin from 2 days before until 3 days after contrast administration. Three days after the procedure both control and study arms were given a statin. All patients received intravenous volume expansion and iso-osmolar contrast media. The incidence of CI-AKI was lower in the rosuvastatin-treated group (2.3 versus 3.9%, p=0.01). Although this benefit was consistently observed among the various subgroups studied, it was more significant in patients with CKD stage 2. There were no significant differences in all-cause deaths or need for kidney replacement therapy. Importantly, there were no significant differences between the two groups in rates of muscle pain and liver function tests. Recent meta-analyses74,75 have also shown a significant reduction in the incidence of CI-AKI associated with statin therapy.
ConclusionIt seems reasonable and safe to start a statin prior to angiographic procedures among those patients who are likely to be started on such a drug prior to discharge, regardless of baseline kidney function. Nevertheless data on CKD patients have been conflicting and exclude patients with CKD stage 4.
Removal of nephrotoxic drugsPotential nephrotoxic drugs should be discontinued, whenever possible, before contrast administration (aminoglycosides, vancomycin, amphotericin B, cisplatin, non-steroidal anti-inflammatory drugs).
Some guidelines suggest that metformin ought to be discontinued at least 12h before contrast administration and not be resumed for a minimum a 36h after the procedure. The rational is that the onset of CI-AKI is quite rapid and metformin retention in this setting can lead to lactic acidosis. This recommendation is based on observational studies, case reports and case series.76
The chronic use of angiotensin-converting-enzyme inhibitors (ACEi) or angiotensin receptor blockers (ARB) prior to contrast studies was associated with a higher incidence of CI-AKI in a retrospective study.17 Nevertheless, a previous study enrolling 220 patients who were on ACEi and/or ARB had shown no benefit in the group of patients in which these drugs were withheld compared to a control group in which these agents were continued.77 Whether or not the discontinuation of these drugs reduced the risk of CI-AKI is still a matter of debate. At present there is insufficient evidence to recommend discontinuation of ACEi and ARB prior to contrast administration.78
Extracorporeal blood purificationRationalIodinated contrast is water soluble and freely filtered in the glomeruli so that its elimination is slower in patients with chronic kidney disease. It is also efficiently cleared by hemodialysis and to a lesser extent, hemofiltration.79 Therefore it could be that removal of contrast media immediately after the procedure could effectively prevent CI-AKI.
EvidenceRCTs enrolling patients with variable degrees of CKD scheduled for coronary angiography have shown a reduction of the incidence of CI-AKI in those submitted to pre- and post-procedure hemofiltration80,81 or hemodialysis.82 Clinically meaningful outcomes as need for renal replacement therapy (RRT) due to oliguria,80,82 in-hospital mortality,80 need for chronic dialysis80,82 and mortality at 1 year80 were significantly better in the hemofiltration80/hemodialysis82 arm compared with intravenous volume expansion in the control arm. However, both of these trials were not blinded and patients in the RRT group were admitted to an intensive care unit whereas patients in the control arm were in a step down-unit, with a less intensive care. Creatinine removal by the procedure can also explain some of the early differences in outcomes. Moreover, one trial81 comparing the effect of intravenous volume expansion alone versus hemofiltration only after the procedure versus hemofiltration done before and after contrast administration showed that it is necessary for patients to undergo hemofiltration before the procedure to obtain the full clinical benefit. This suggests that more important than contrast removal by RRT, high-volume controlled hydration before contrast media exposure may play a major role in preventing CI-AKI.
Despite the benefits observed in these trials, meta-analyses have shown no benefit of RRT in the prevention of CI-AKI. Moreover, if we limit the analysis to studies of hemodialysis, it is associated with an increase in the risk of CI-AKI.83
There is no evidence that hemodialysis immediately after intravascular contrast administration prevents volume overload or decline of residual renal function in stage 5d CKD patients.84–86
ConclusionThe only studies we have available are small and flawed. Moreover meta-analyses have shown no benefit or even harm in the use of RRT for the prevention of CI-AKI. Therefore this is not a recommended strategy for the prevention of CI-AKI.
In stage 5 CKD patients on hemodialysis, there is no evidence of the benefit of immediate dialysis after intravascular contrast administration.
ConclusionCI-AKI is a frequent cause of intrahospital acute kidney injury and one of the most common complications of iodinated contrast administration. The most important risk factors for CI-AKI are pre-existing kidney dysfunction, particularly in association with diabetes mellitus and intravascular volume depletion. As for most forms of AKI there is no specific treatment once it is established. Nevertheless it is one of the few predictable forms of AKI, giving us a unique opportunity for identification of patients at high-risk and institution of prophylactic interventions. Most trials addressing prophylactic strategies for CI-AKI enroll patients subjected to intra-arterial administration of iodinated contrast media, usually for coronary or peripheral angiography. The use of non-ionic iodinated contrast media, either low- or iso-osmolar, seems to reduce the risk of CI-AKI. Innumerous pharmacological and non-pharmacological interventions have been tested, of which the most successful and widely accepted is volume expansion with intravenous isotonic fluids, either isotonic saline or isotonic sodium bicarbonate. Also, oral NAC is frequently used in the prevention of CI-AKI based on its possible benefit and favorable side effects profile. Recently, some studies have also shown the benefit of the pre-treatment with statins.
Based on the available evidence, the authors present a practical approach to the prevention of CI-AKI in Fig. 1. First, the risk of CI-AKI should be assessed, based on the presence and combination of the most important risk factors, namely baseline kidney function, diabetes mellitus and the type of procedure scheduled. High-risk patients, that is those with baseline estimated glomerular filtration rate <60mL/min/1.73m2, should be subjected to prophylactic measures of which the most important are intravenous hydration and oral N-acetylcysteine. Also the lowest possible dose of non-ionic contrast media should be administered.
Conflicts of interestThe authors have no conflicts of interest to declare.