The leiomyosarcoma of the inferior vena cava (IVC) is a rare clinical entity, although it represents the most common malignant tumor of the venous system. Level II IVC tumors (supra-renal) are the most frequent and those who have a better prognosis for the development of symptoms earlier.
Case reportThe authors report a case of IVC leiomyosarcoma in a 59-year-old woman, presenting with DVT of the right lower limb, subsequent to prolongued nonspecific abdominal pain. Computed tomography revealed a large retroperitoneal neoformation, centered to IVC, which extended above the renal veins (the left one patent and the right one involved in the mass). The patient underwent en block resection of the tumor and reconstruction of the renal veins: construction of a new IVC bifurcation at the supra-renal level with a bifurcated PTFE graft, followed by graft extension to both renal veins using externally-supported 8mm PTFE grafts. Histology revealed a high-grade leiomyosarcoma. The postoperative period was complicated by a type 2 MI and retroperitoneal hematoma, with occlusion of the right graft branch and partial infarction of the right kidney. The patient underwent surgery again and proceeded to partial resection of the thrombosed graft branch. The patient was discharged home under anticoagulation and is clinically well without edema of the lower limbs, normal renal function, and has begun adjunctive therapy.
ConclusionThe prognosis of these tumors is poor, with a high recurrence rate. An aggressive surgical approach combined with adjuvant therapy may not be curative, but is the best strategy to prolong survival.
O leiomiossarcoma da veia cava inferior (VCI) é uma entidade clinica rara, embora represente o tumor maligno mais comum do sistema venoso. Os tumores do segmento II da VCI (supra-renal) são os mais frequentes e aqueles que apresentam melhor prognóstico pelo desenvolvimento de sintomatologia mais precocemente.
Caso clínicoOs autores apresentam um caso de leiomiossarcoma da VCI numa doente de 59 anos, que se manifestou por TVP do membro inferior direito, subsequente a quadro de dor abdominal inespecífico arrastado. A tomografia computorizada revelou volumosa neoformação retroperitoneal, centrada à VCI, que se estendia acima das veias renais (a esquerda permeável e a direita envolvida na massa). A doente foi submetida a ressecção em bloco do tumor e reconstrução das veias renais: construção de neo-bifurcação da VCI com prótese de PTFE bifurcada seguido de reconstrução de ambas as veias renais com próteses aneladas (PTFE-8). A histologia revelou tratar-se de um leiomiossarcoma de alto grau. O pós-operatório foi complicado por EAM tipo 2 e por hematoma retroperitoneal com oclusão do ramo protésico direito e enfarte parcial do rim direito. Foi reintervencionada, tendo-se procedido à ressecção parcial do ramo protésico trombosado. A doente teve alta anticoagulada e encontra-se clinicamente bem, sem edema dos membros inferiores, com função renal normal, e iniciou terapêutiva adjuvante.
ConclusãoO prognóstico destes tumores é reservado, com elevada taxa de recorrência. Uma estratégia cirúrgica agressiva combinada com terapêutica adjuvante pode não ser curativa, mas constitui a melhor estratégia para prolongar a sobrevida.
The IVC leiomyosarcoma is a rare clinical entity, with only 300 cases described in the literature and most of them refer to individual case reports or small series.1–5 Represents the tumor that most often affects the venous system and is associated with a poor prognosis.1,3,4
Most patients are asymptomatic, for it is a slow growth tumor and only manifests symptoms in advanced stages of the disease.4,6–8 Its incidence is more common in women around the sixth decade of life.1 Abdominal pain is the most common symptom; edema of the lower limbs, back pain, weight loss, fever, palpable abdominal mass and, rarely, Budd–Chiari syndrome can also be part of the clinical presentation spectrum.5,9–11
The treatment of this tumor is still controversial. In this paper, we present a case report of a patient submitted to a complex vascular construction.
Case reportA 59-year-old woman presented to her attending physician with epigastric and lower back right pain, associated with constipation and weight of the lower limbs. The abdominal and renal ultrasound scan was normal. Six months later, she developed pain and asymmetrical edema of the right lower limb, with tenderness of leg muscle mass and positive Homan's sign. She maintained pain in the epigastric and right upper quadrant on deep palpation and no palpable masses were felt. The patient did not have fever, chest pain, gastrointestinal, urinary or respiratory changes, as well as recent weight loss. She was admitted to a local hospital with the diagnosis of iliofemoral deep vein thrombosis of the right lower limb.
Her past medical and surgical history included essential hypertension, past caesarean section and laparoscopic cholecystectomy; no known risk factors for venous thrombosis or relevant family history were present.
The venous Color-flow Duplex Scan revealed thrombus extension to the juxta-renal IVC and a nodular mass was observed at this level. An abdominal and pelvic CT scan was performed, revealing the presence of a solid heterogeneous mass in the topography of the duodenal arch, without clear cleavage plane with the duodenum, the uncinate process of the pancreas and the IVC. The examination was completed by ecoendoscopy, which was suggestive of GIST, and ultrasound-guided biopsy of the lesion, which was inconclusive. The analytic study was negative for both pro-thrombotic factors and tumor markers.
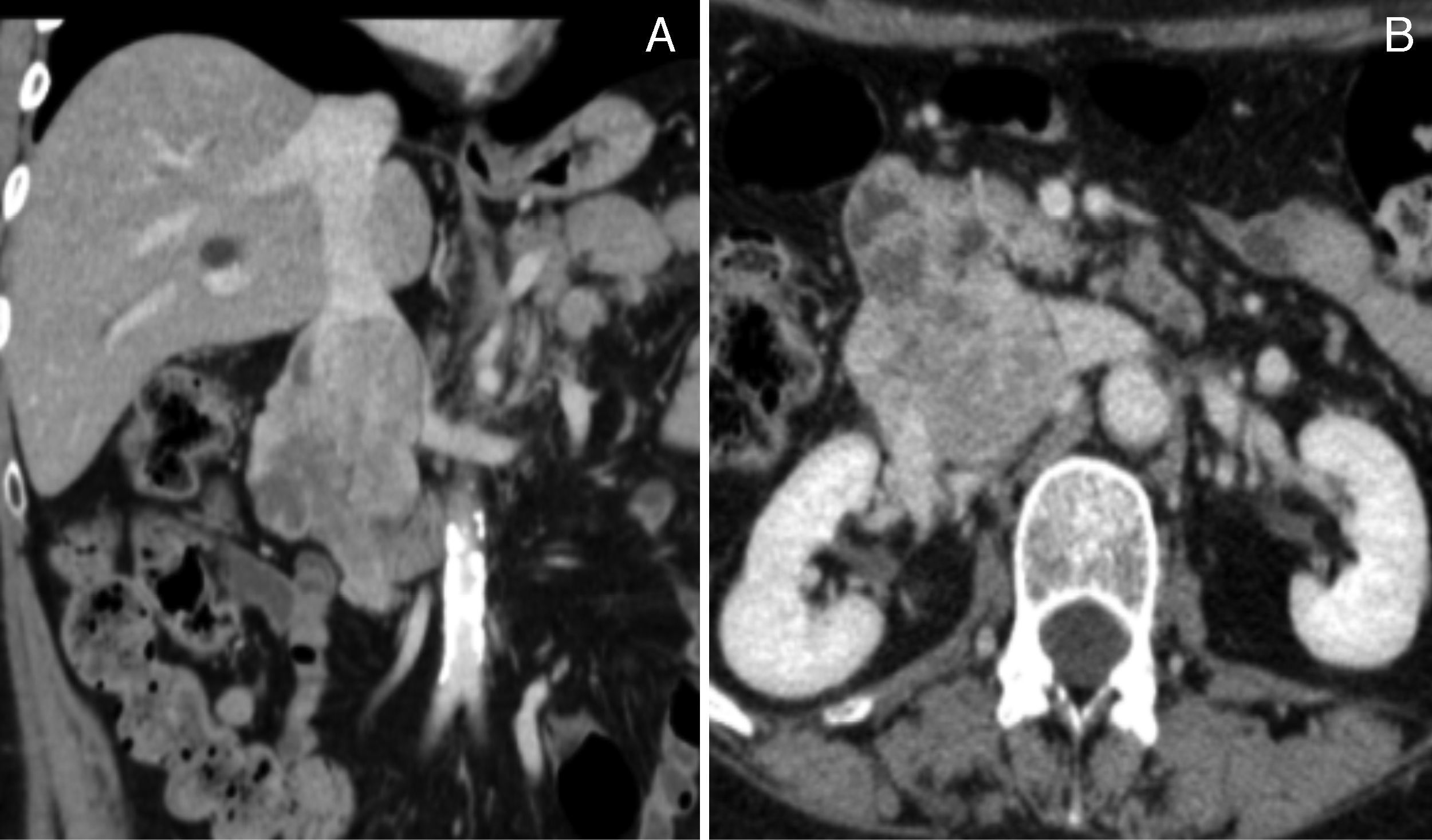
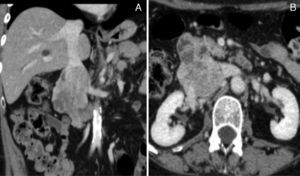
Given the improvement of the right lower limb edema, the anticoagulation therapy was interrupted after 3 months. Then, an abdominal and pelvic Angio-CT scan was requested which allowed a better characterization of a neoplasm centered to the IVC (Fig. 1A and 1B), suggestive of a IVC leiomyosarcoma and no other pathological findings were found.
(A) and (B) Contrast CT scan demonstrating a large neoformation centered to the IVC at the renal veins level, with heterogeneous uptake of iodinated contrast media and about 8cm long axis. It presents expansive characteristics and extends beyond the boundaries of the vein, losing cleavage surface with the duodenal arch, but without reaching the pancreas. There is thrombosis of infra-renal IVC and right common iliac vein, and patent renal veins.
The patient was then referred for surgery, after a multidisciplinary planning, involving Oncology, General Surgery and Vascular Surgery, and was admitted in the Vascular Surgery Department.
Pre-operatively, a flebogram was performed and showed occlusion of the infrarenal IVC and both common iliac veins.
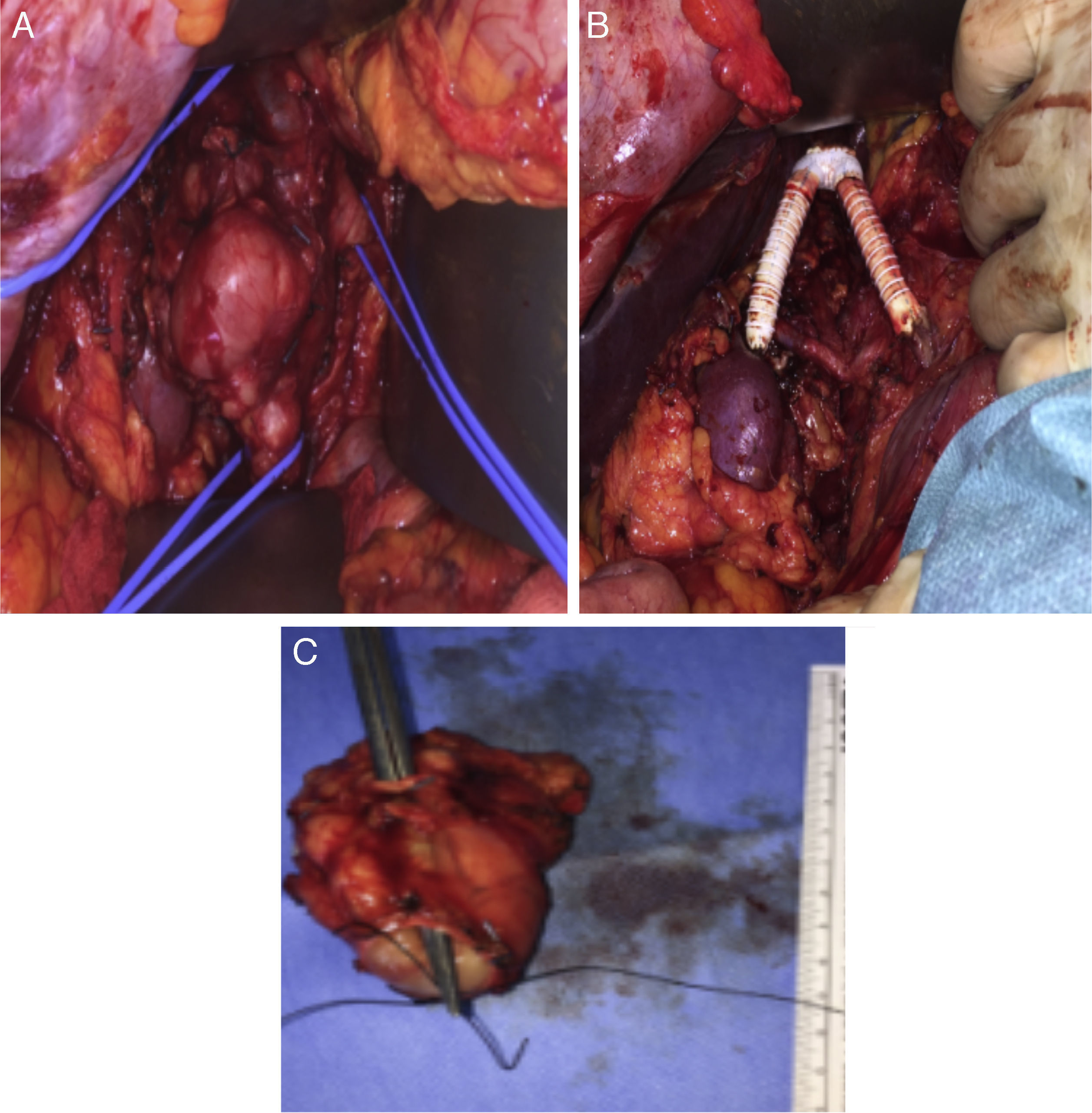
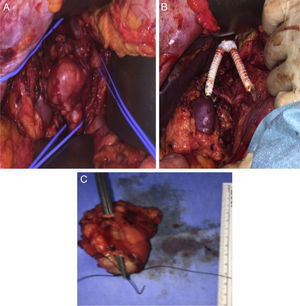
The surgical approach was conducted through a large right subcostal incision. The supra-renal IVC was identified and was free of disease, as well as the left renal vein. The lower segment of the IVC was occluded (fibrotic) and the mass was close to the right renal hilum. After section of the proximal and distal IVC and both renal veins, the en block resection of the tumor was carried on. The technique for renal veins reconstruction included the construction of a new IVC bifurcation at the supra-renal level with a bifurcated 18mm×9mm PTFE graft, followed by graft extension to both renal veins using externally-supported 8mm PTFE grafts (Fig. 2A–C).
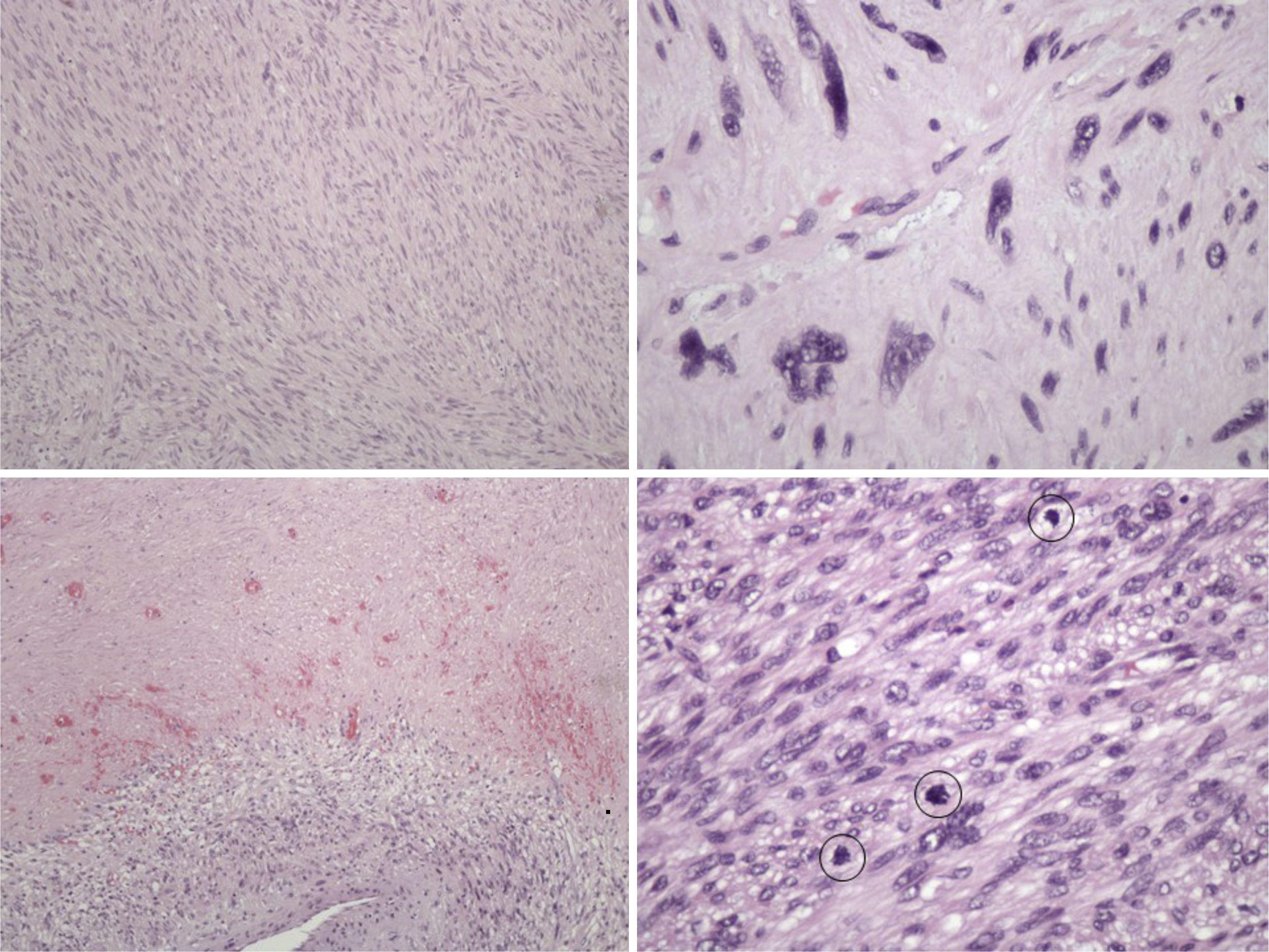
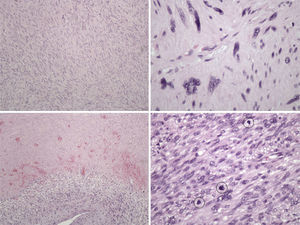
The pathology examination revealed a high-grade leiomyosarcoma, with clear margins (Fig. 3).
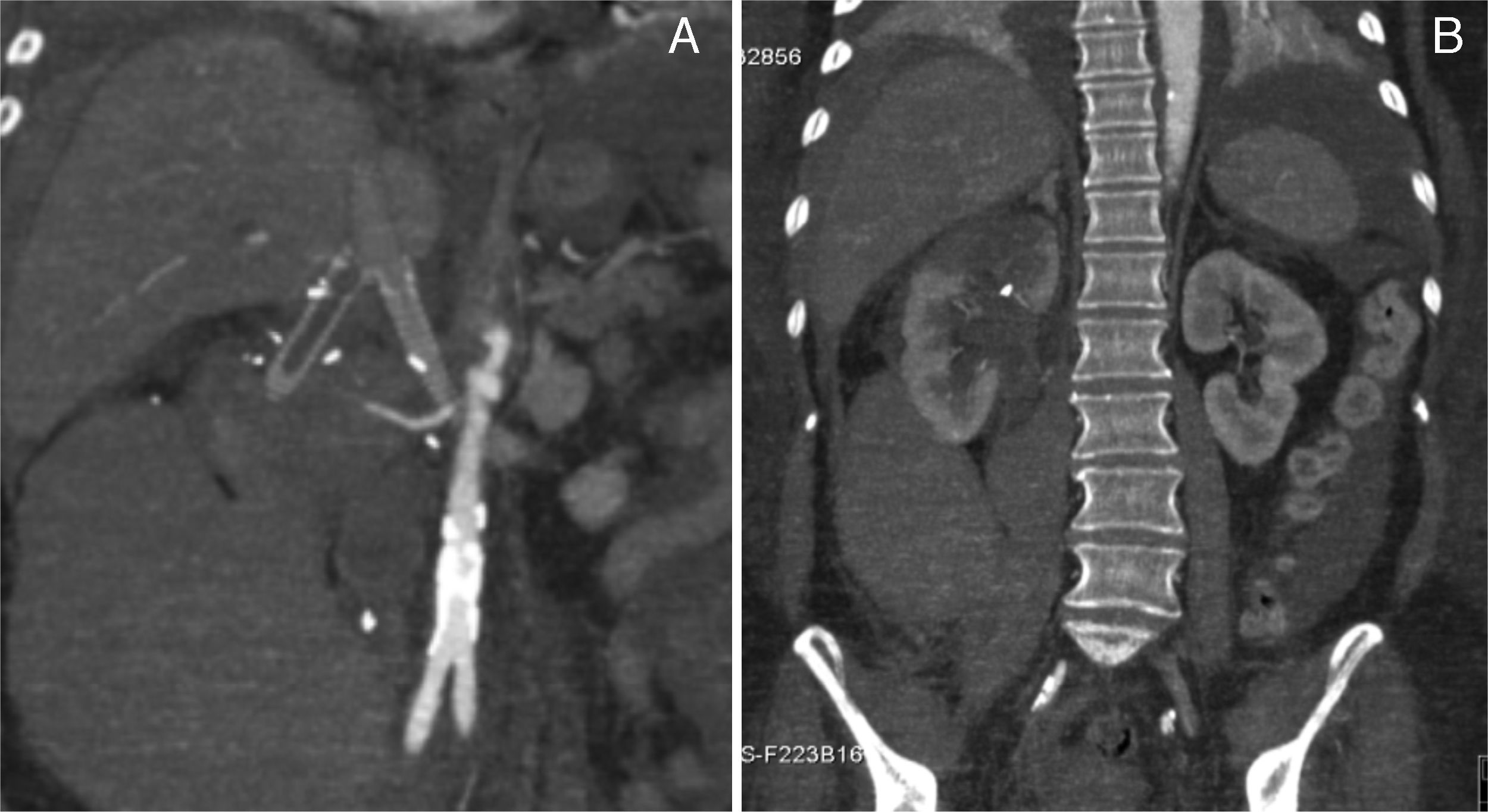
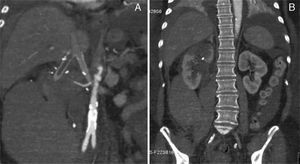
The postoperative period was complicated by a type II miocardial infarction (2 day PO) needing to start antiplatelet and anticoagulation therapy. Subsequently, by day 5 PO, the patient complained upper abdominal and back pain associated to an acute anemia of 6.6g/dl. The emergency CT scan revealed an extensive right retroperitoneal hematoma, thrombosis of the right reno-caval limb graft and partial infarction of the ipsilateral kidney (Fig. 4A and B). The left kidney reconstruction was patent.
The patient was then submitted to laparotomy for hemostasis. As most of the right kidney surface did not appear ischemic, we did not proceed with nephrectomy, and ligation and removal of the right kidney venous graft (thrombosed) was performed.
The patient was discharged by the 20th day PO, under anticoagulation and is clinically well and with resolution of pain and edema of the lower limbs. The renal function is normal. She is now under adjunctive treatment at the Oncology Department.
DiscussionAnatomically, the IVC is divided into three levels, each one associated with different clinical manifestations and surgical considerations. Level I is the infra-renal IVC, level II is the para-renal, supra-renal and infra-hepatic IVC and Level III is the supra-hepatic.6
Level II tumors are the most frequent and those who have a better prognosis for the development of earlier symptoms. Approximately 50% of the cases present with metastasis.1,9
Level I tumors presentation includes back and abdominal pain, edema of lower limbs and, rarely, deep venous thrombosis.12 Lower limb edema occurs more frequently associated with deep vein thrombosis than with occlusion of the IVC by the tumor.4,13,14 In level I neoplasms, patients complains are pain in the right upper abdominal quadrant and, when reaching the renal veins, renovascular hypertension or kidney failure may be found. The Budd–Chiari syndrome is a typical expression of level III tumors, when there is thrombosis of the hepatic veins.12
With regard to diagnosis, contrast CT scan and MRI are the main diagnostic tools. They allow the characterization of the tumor origin, the inferior vena cava involvement and obstruction as well as the collateral circulation. They may demonstrate contiguous invasion, assess the resectability and exclude the existence of extra-abdominal metastases. The definitive histological diagnosis can be obtained by US or CT-guided biopsy.3,8 The use of PET also contributes to evaluate the existence of metastasis and flebography, presently less used, allows to assess the degree of IVC obstruction and its main branches, thus being important for surgical planning.5
Even with all these diagnostic modalities, in many cases it is not possible pre-operatively to determine precisely the origin of these tumors. Moreover, the anatomic location can make it difficult to perform a percutaneous biopsy. Often, the surgeon is faced with the challenge of making an exploratory laparotomy with a tumor of unknown origin and histology.2
The preferred surgical approach, particularly for tumors of level II, is through a subcostal incision, which can be improved by lumbotomy or controlateral extension. Level I tumors can also be approached by median laparotomy, while level III tumors require a thoraco-abdominal approach.
Surgical resection follows the principles of surgical oncology-en block resection with negative margins. Complete resection of the tumor is often difficult because of its location and the involvement of adjacent organs and vascular structures. Thus, 1/3 to half of the patients show locally recurrence or metastization.1,6,15 In general, efforts for venous reconstruction decrease the post-operative morbidity16 and the need and type of reconstruction are influenced by three major factors: (1) the level of IVC affected and the involvement of the renal veins; (2) the extent of the IVC resection (partial or circumferential); (3) the presence of colateral venous flow.8
In level I and II lesions, with chronic occlusion and developed colateral venous flow, IVC ligation without reconstruction is usually well tolerated.15–17 If the occlusion of the IVC occurs acutely, the lower limb edema may be a post-operative issue in 36–70% of the patients and about 1/3 will develop chronic edema or post-thrombotic syndrome.16
In level II tumors, when ligation of the right renal vein is needed, a right nephrectomy is also performed by most authors, even if the kidney is not directly involved, for the absence of adequate colateral venous outflow determining a high probability of ischemia.2 In the case reported we aimed at kidney salvage by bilateral venous reconstruction, as the tumor did not invade the renal hilum structures. In left sided tumors, the left renal vein may be ligated after the emergence of the suprarenal and gonadal veins which are often enough to maintain an adequate venous drainage.
Techniques for IVC reconstruction include a direct suture or patch angioplasty when there is <75% circumference involvement of the IVC, that enabling partial resection.14 Prosthetic reconstruction is more common, and must be performed in the absence of adequate collateral venous flow with risk of developing renal failure or extreme lower limb edema.2,6 Kieffer et al. pointed out a cut-off value for the proximal pressure in the IVC of 30mmHg; above this level, there is excessive venous pressure justifying reconstruction.
In level II tumors, the venous drainage of the kidneys is a main issue, especially in cases of poor renal function and/or lack of adequate venous drainage of the left kidney. So, it can be appropriate to carry out bilateral venous reconstruction, whenever possible.7 Various techniques are suggested in the literature including anastomosis of the left renal vein to the inferior mesenteric vein or right kidney auto-transplantation.2,6,7,11
In the present case we report an original technical solution for bilateral venous reconstruction.
Peri-operative mortality and morbidity range between 0–25% and 18–50%, respectively. These values are related to the magnitude of the vascular resection and to the reconstructions performed.4,6 Cardiopulmonary disease, liver and/or kidney failure, lower limb edema, graft occlusion and infection are reported as the major early complications.16 In the series presented by Fiore et al, there is no relationship between graft thrombosis and the type of graft with the subsequent development of renal failure.8 Graft occlusion varies between 7% and 28%4,8 and strategies to prevent thrombosis and improve patency include ringed PTFE grafts, oral anticoagulation and the construction of arteriovenous fistula in the femoral region.2,3
IVC leiomiosarcoma is an aggressive disease and complete surgical resection provides the only chance of cure or palliation of symptoms.16,18 Patients undergoing complete resection had free disease survival rates of 76% and 33% at 3 and 5 years, respectively. When the resection is incomplete, the survival rate is near zero at 3 years.4 In the international registry by Mingoli et al., predictors of increased mortality are level III tumors, Budd–Chiari's syndrome, edema of the lower limbs, intraluminal growth and occlusion of the IVC; level II tumors, the absence of palpable abdominal mass and abdominal pain are associated with better outcome and long-term survival.1
Histologically, leiomyosarcoma is a malignant neoplasm showing pure smooth-muscle differentiation. Macroscopy forms a gray to white to tan mass, with a whorled appearance; the tumor border appears well-circumscribed. The typical histological pattern is that of intersecting, sharply marginated fascicles of spindle cells.19–21 In this case, there are zones with coagulative tumor necrosis. The tumor-cell nuclei are characteristically elongated and blunt-ended; pleomorphisms are notable and mitotic figures are seen. The cytoplasm is eosinophilic to pale. Immunophenotype: smooth-muscle-actin (SMA), desmin and h-caldesmon are positive.
Adjuvant therapy was considered ineffective in the treatment of IVC leiomyosarcomas for many years. Currently, it is accepted that patients undergoing adjuvant chemotherapy and/or radiotherapy have better survival rates,2,7,16 even when local recurrence or distance metastasis are present. So, presently, aggressive surgical approach combined with adjuvant therapy provides the best treatment strategy for patients with IVC leiomyosarcoma.6,7
Close surveillance with regular CT scans searching local recurrence or distance metastasis is indicated. However, there are no defined follow up intervals, which should be requested according to the individual risk. Metastatic disease occurs more frequently in the lung.
ConclusionThe authors report a case of level II IVC leiomyosarcoma submitted to radical en bloc resection associated to an original technique for bilateral venous reconstruction.
Ethical responsibilitiesProtection of people and animalsThe authors state that for this investigation there has been no experience in humans and/or animals.
Confidentiality of dataThe authors state that they followed the protocols of their work center on the publication of patient data.
Right to privacy and consent in writingThe authors declare having received written consent from patients and/or subjects mentioned in the article. The corresponding author must be in possession of this document.
Conflicts of interestThe authors have no conflicts of interest to declare.