A systemic inflamatory response is common after EVAR. Its clinical impact is unknown, and although is it usually well tolerated, there is concern it might be associated with increased morbidity and mortality in high risk patients. This study aims to evaluate the occurrence of the post-implantation syndrome (PIS) in patients undergoing EVAR, its characteristics and clinical significance.
MethodsThis study is a retrospective observational analysis of patients undergoing elective EVAR between November 2012 and November 2014. PIS was defined by fever (>38°C) and leukocytosis (>12,000μL−1), excluding infectious complications. We evaluated the epidemiological characteristics of the patient, aneurysm and procedure characteristics and their relationship with development of PIS.
ResultsFifty-two patients were included, and 21.2% were diagnosed with PIS. The ePTFE grafts were not associated with the occurrence of the syndrome, in contrast with polyester stent grafts (0% vs. 28.2%, p=0.031). The age and gender of patients, the diameter of the aneurysm, duration and radiation dose and the configuration of the stent graft (aorto-bi-iliac, aorto-uni-iliac or fenestrated) were not associated with PIS. There was no statistically significant difference in the occurrence of major cardiovascular events during hospitalization in both groups.
ConclusionThe inflammatory syndrome after EVAR occurs in a significant percentage of patients (21%). Stent grafts constructed by polyester are a significant risk factor. Despite the exuberant inflammatory response, it was not associated with increased occurrence of cardiovascular events, and it is usually benign, well tolerated and self-limiting.
É frequente uma resposta inflamatória sistémica após EVAR. O seu impacto clínico é desconhecido, e apesar de habitualmente ser bem tolerado existe o receio de estar associado a maior morbi-mortalidade em doentes de alto risco. Este estudo tem como objectivo a avaliação da ocorrência do síndrome pós-implantação (SPI) nos doentes submetidos a EVAR, suas características e relevância clínica.
Material e métodosEstudo observacional retrospectico de doentes submetidos a EVAR electivo entre Novembro de 2012 e Novembro de 2014. O SPI foi definido por febre (>38°C) e leucocitose (>12.000/μL), excluídas complicações infecciosas. O outcome primário foi o diagnóstico de SPI. Foram avaliadas as características epidemiológicas do doente, características do aneurisma e do procedimento e sua relação com o SPI.
ResultadosForam incluídos 52 doentes, dos quais 21,2% desenvolveram SPI. As endopróteses de ePTFE não foram associadas à ocorrência de SPI, comparativamente às de poliéster (0% vs. 28,2%, p=0.031). A idade e o sexo dos pacientes, o diâmetro do aneurisma, o tempo e a dose de radiação e a configuração da endoprótese (aorto-bi-ilíaca, aorto-uni-ilíaca ou fenestrada) não foram associados à ocorrência do síndrome. Não se observaram diferenças estatisticamente significativas na ocorrência de eventos cardiovasculares major durante o internamento em ambos os grupos.
ConclusãoO SPI ocorre numa percentagem significativa de doentes (21%). As endopróteses construídas com poliéster são um factor de risco significativo. Apesar da resposta inflamatória exuberante, esta não se associou à ocorrência de eventos cardiovasculares, sendo habitualmente benigna, bem tolerada e auto-limitada.
After implantation of a stent graft in endovascular repair of abdominal aortic aneurysm (EVAR) it is common a systemic inflammatory response, which sometimes is exuberant. The inflammatory syndrome after EVAR, or post-implantation syndrome (PIS), is defined by fever and leukocytosis in the postoperative period when infectious complications are excluded.1,2 Despite being described since 1999,2 the etiologic mechanisms, its significance and its consequences are yet not fully understood. The incidence differs in the published studies, in part due to different diagnostic criteria, and it is estimated from 14% to 60%.1–6 The inflammatory reaction is attributed to endothelial dysfunction in contact with the graft material.7 Acute thrombus formation in the aneurysmal sac may also contribute.7 The clinical impact of this inflammatory response is unknown, and although it is usually well tolerated and self-limiting and there is concern that it could be associated with worse prognosis and higher morbidity and mortality in high cardiovascular risk patients. The necessity of treatment and the most appropriate type of treatment remains controversial.
This study aims to evaluate the occurrence of post-implantation syndrome in our patients submitted to elective endovascular repair of abdominal aortic aneurysm, its characteristics and its clinical significance.
Materials and methodsThis study is a retrospective observational analysis of the abdominal aortic aneurysms treated by elective EVAR between November of 2012 and November of 2014. All patients undergoing EVAR consecutively were included. Infectious aneurysms, ruptured aneurysms and false aneurysms were excluded from the analysis. We proceed to systematically review the electronic clinical records, with evaluation of the epidemiological characteristics of patients (age and gender), auricular temperature and analytical parameters (erythrocytes, leukocytes, platelets, c reactive protein (CRP)), before and after the procedure. The temperature evaluation was performed on the patient admission to the hospital, and after that, at every standard ward shift (every 6h). Analytical blood evaluation was performed at patient's admission to the hospital, on the first postoperative day, and after that, at variable timing, by decision of the treating physician. Aneurysm features diameter, location), characteristics of stent graft (configuration of the endoprosthesis (aorto-uni-iliac, aorto-bi-iliac, fEVAR), manufacturer, model and construction material) and the procedure approach used (percutaneous or surgical cutdown femoral access) were also registered. The radiation dose, the fluoroscopy time of the intervention and the length of stay (total hospital stay and on intermediate care unit) were analyzed. Major complications and the occurrence of cardiovascular events (myocardial ischemia with EKG signs or symptomatic elevation of myocardial ischemia markers or acute cerebrovascular events) in the postoperative period were also recorded.
The post-implantation syndrome was defined by the presence of leukocytosis (>12,000 leukocytes/μL) plus the occurrence of fever (>38°C – auricular temperature) during the postoperative period when infectious complications were excluded (no clinical evidence of infection, negative blood cultures, absence of local complications of the surgical wound).1 All patients included were treated by the same surgical and anesthesiology team in an operating theater room, in an University Hospital Center. All patients fulfilled the criteria of suitability for endovascular repair defined in the literature.8 Prophylactic antibiotic (cefazolin, 2g) was routinely administered to all patients before the procedure. The stent-grafts used were Endurant II (Medtronic Inc®, Santa Rosa, CA, USA), Zenith Fenestrated Endovascular Graft (Cook Inc.®, Indianapolis, IN, USA) and Excluder (WL Gore & Associates®, Flagstaff, AZ, USA). The first two were constructed with polyester, and the latter is constructed with ePTFE. Atrium Advanta V12 covered stents (ePTFE covered) were used in the fenestrated procedures.
The statistical treatment of data was performed with SPSS Statistics 21.0® (IBM). The evaluation of the statistical significance of the differences found was performed with Qui2 tests, Student's t test and Fisher exact test when appropriate. Statistical significance was considered for differences of 0.05.
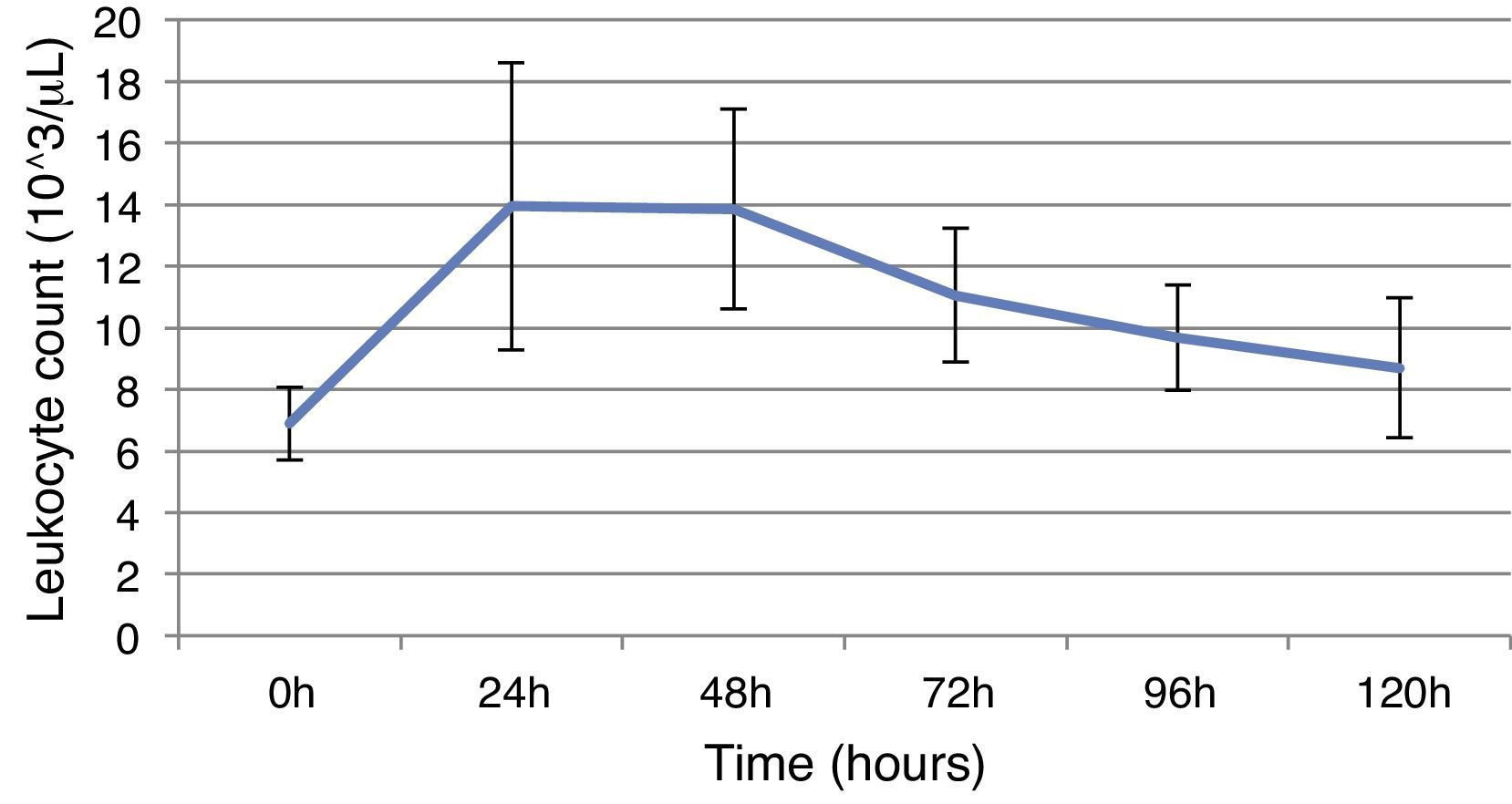
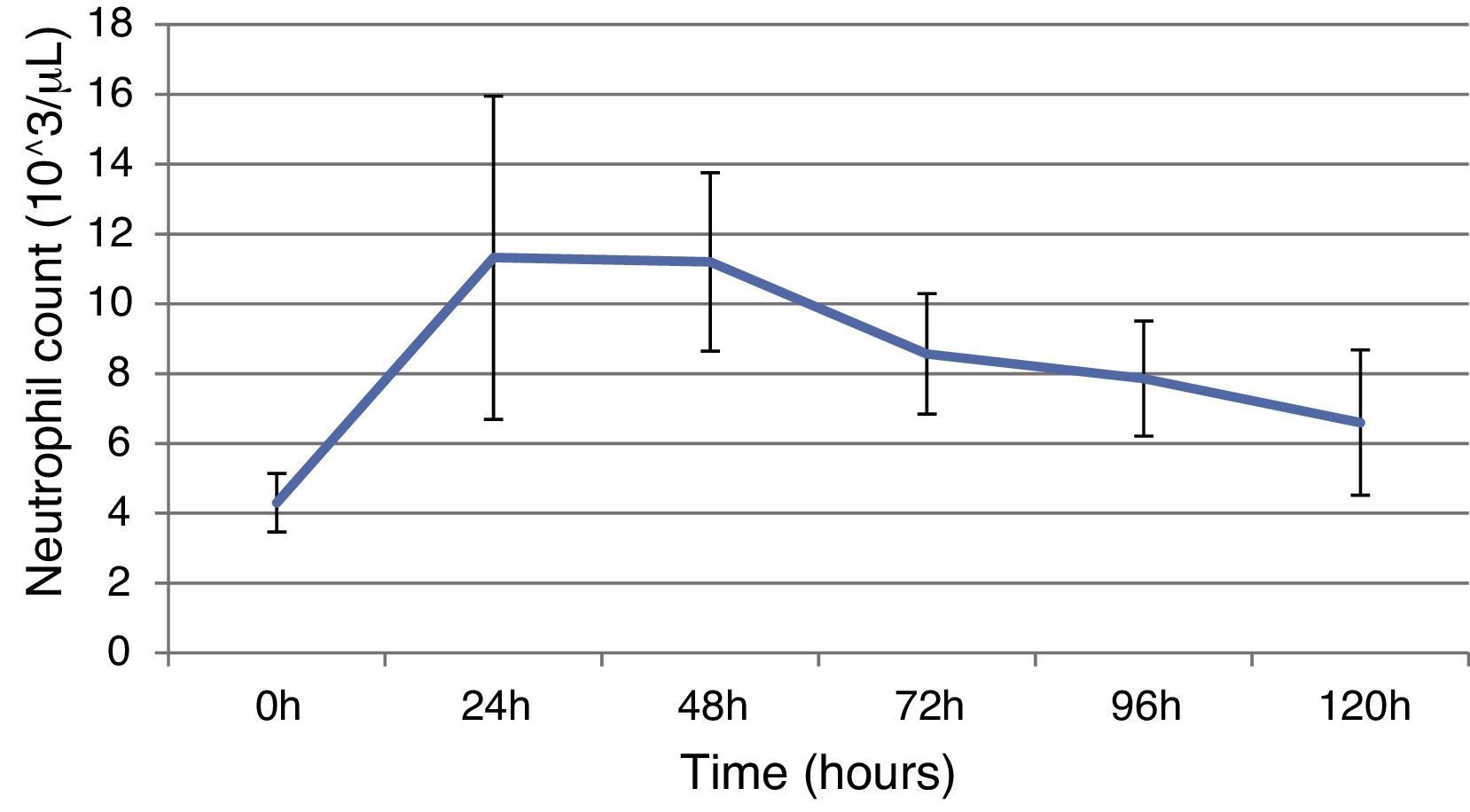
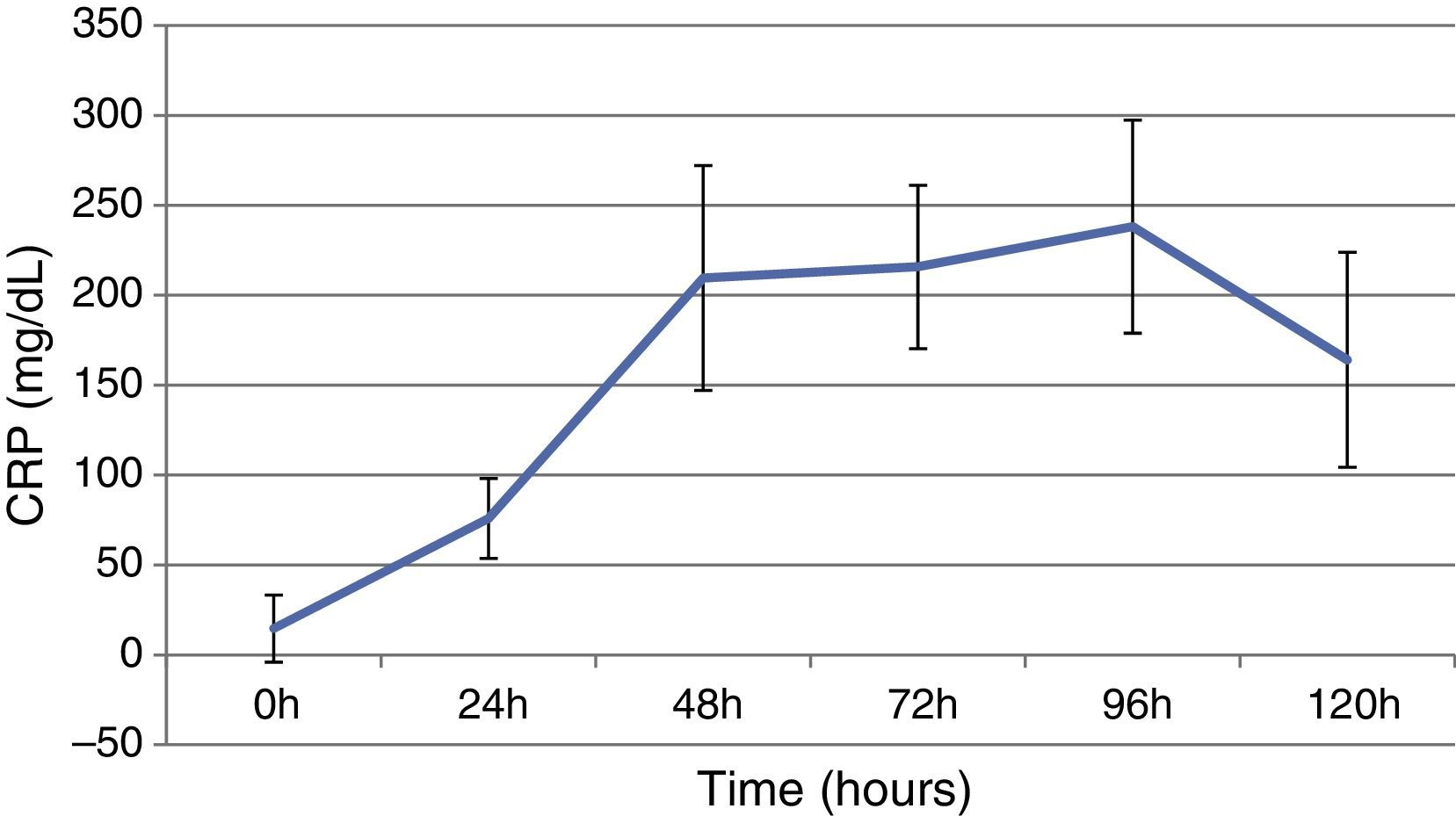
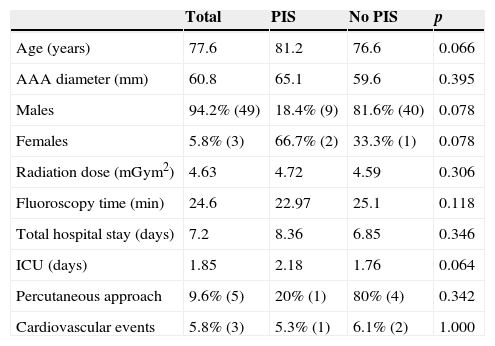
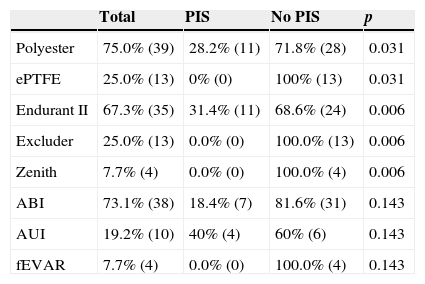
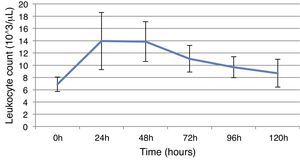
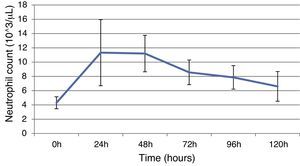
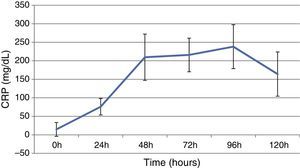
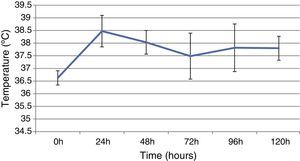
ResultsThis study included 52 patients with an average age of 77.6 years (standard deviation 9.4, minimum 58, maximum 94) and 94.2% were male (Table 1). Of these patients, 21.2% developed post-implantation syndrome. On the post-operative period, 44.2% of the patients had leukocytosis. The development of leukocytosis and neutrophilia had a rapid rise and peak in the first 24h after implantation of the stentgraft, with a slow and steady decline thereafter (Graphs 1 and 2). The increase in CRP was slower, with peak at 96h (Graph 3). Fever affected more than half of patients (55.7%). The temperature increased rapidly, peaking within 24h, and remained high over the next few days (Graph 4).
Sample characteristics.
| Total | PIS | No PIS | p | |
|---|---|---|---|---|
| Age (years) | 77.6 | 81.2 | 76.6 | 0.066 |
| AAA diameter (mm) | 60.8 | 65.1 | 59.6 | 0.395 |
| Males | 94.2% (49) | 18.4% (9) | 81.6% (40) | 0.078 |
| Females | 5.8% (3) | 66.7% (2) | 33.3% (1) | 0.078 |
| Radiation dose (mGym2) | 4.63 | 4.72 | 4.59 | 0.306 |
| Fluoroscopy time (min) | 24.6 | 22.97 | 25.1 | 0.118 |
| Total hospital stay (days) | 7.2 | 8.36 | 6.85 | 0.346 |
| ICU (days) | 1.85 | 2.18 | 1.76 | 0.064 |
| Percutaneous approach | 9.6% (5) | 20% (1) | 80% (4) | 0.342 |
| Cardiovascular events | 5.8% (3) | 5.3% (1) | 6.1% (2) | 1.000 |
AAA, abdominal aortic aneurysm; PIS, post-implantation syndrome; ICU, intermediate care unit.
The mean diameter of aneurysms was 60.8mm (minimum 40mm, maximum 95mm), and an association between the diameter of the aneurysm and the occurrence of PIS was not observed (Table 1).
Regarding the stent graft morphology, 73.1% were treated with aorto-bi-iliac stent-graft, 19.2% with aorto-uni-iliac stent and 7.7% with fEVAR. Considering the manufacturer of the endoprosthesis, 67.3% were treated with Endurant II, 25.0% with Excluder and 7.7% with Zenith stent-graft. The ePTFE grafts were not associated with the occurrence of the syndrome, on contrary to the polyester endoprosthesis (0% vs. 28.2%, p=0.031). The subgroups treated with Zenith and Excluder stents had no occurrence of PIS, p=0.006 (Table 2). Regarding the procedure approach, the majority of patients (90.4%) were treated with surgical cut down femoral access and 9.6% of patients were treated with percutaneous access, with no statistical significant influence on the occurrence of PIS (Table 1).
Relationship between stent-graft characteristics and PIS.
| Total | PIS | No PIS | p | |
|---|---|---|---|---|
| Polyester | 75.0% (39) | 28.2% (11) | 71.8% (28) | 0.031 |
| ePTFE | 25.0% (13) | 0% (0) | 100% (13) | 0.031 |
| Endurant II | 67.3% (35) | 31.4% (11) | 68.6% (24) | 0.006 |
| Excluder | 25.0% (13) | 0.0% (0) | 100.0% (13) | 0.006 |
| Zenith | 7.7% (4) | 0.0% (0) | 100.0% (4) | 0.006 |
| ABI | 73.1% (38) | 18.4% (7) | 81.6% (31) | 0.143 |
| AUI | 19.2% (10) | 40% (4) | 60% (6) | 0.143 |
| fEVAR | 7.7% (4) | 0.0% (0) | 100.0% (4) | 0.143 |
ABI, aorto-bi-iliac; AUI, aorto-uni-iliac; fEVAR, fenestrated stent-graft; PIS, post-implantation syndrome.
The age and gender of the patients, the diameter of the aneurysm, fluoroscopy time and radiation dose (Table 1), and the morphological configuration of the stent (aorto-bi-iliac, aorto-uni-iliac or fenestrated) had no relationship with the occurrence of PIS (Table 1). The development of the inflammatory syndrome was associated with a higher total hospital stay (8.63 vs. 6.85 days, p=0346) and on intermediate care unit (2.18 vs. 1.76 days, p=0.064), although with no statistically significant difference (Table 1).
With regard to the analytical data, patients who developed PIS had higher baseline CRP, compared to patients who did not develop the syndrome (11.92mg/dL vs. 4.36mg/dL, p=0.005). There were no other associations between PIS and the base-line analytical data.
There were no statistically significant differences in the occurrence of major cardiovascular events during hospitalization in both groups (5.3% vs. 6.1%, p=1.000) (Table 1).
DiscussionIn this study we found a significant incidence of PIS (21%) in patients undergoing EVAR. This incidence rate is in agreement with the published literature, which varies from 14% to 60%.1–6 This variation is in part explained by different diagnostic criteria. Some authors use as criteria, the development of fever and CRP elevation, while others authors use as criteria, the occurrence of fever and leukocytosis, with different cut-off values for each of these variables.
The stent-graft construction material appears to be the major risk factor for the development of PIS.9,10 In this study, no patient treated with ePTFE graft fulfilled our criteria of PIS. Thus, the occurrence of fever or leukocytosis in patients with ePTFE grafts should raise the suspicion of other complications and trigger off the adequate investigation. We found no PIS on patients treated with the Zenith stent-graft, which is also constructed with polyester. This might be explained by the low number of cases treated with this stent in our series.
The occurrence of PIS led to prolongation of the time of total hospital stay and in intermediate care unit, although without statistically significant differences. The exuberant inflammatory response and clinical concern of a possible infection may be responsible for prolongation of the hospitalization and maybe the unnecessary prescription of antibiotics and unnecessary diagnostic tests, associated with increased risks and health-care costs. Procalcitonin is a biomarker of bacterial infection and sepsis. A prospective pilot study demonstrated the effectiveness of procalcitonin assay in peripheral blood to differentiate the PIS and infection.11 Thus the determination of procalcitonin can help to avoid unnecessary treatment and clinical investigation.
We found no association between the development of PIS and the occurrence of major cardiovascular events. Activation of inflammatory signaling pathways with the consequent release of pro-inflammatory cytokines, leukocyte activation and proliferation, may be associated with increased postoperative morbidity and mortality, especially in high risk patients.12,13 A prospective study that evaluated the occurrence of PIS in patients undergoing EVAR showed that the magnitude of inflammation (measured by the maximum value of high sensitivity CRP) correlated with adverse cardiovascular events within 30 days after the procedure.1 Due to the retrospective design of the study, it was not possible to investigate the presence of asymptomatic elevation of myocardial ischemia markers and its relation to PIS.
The necessity of treatment is controversial. Some authors suggest the use of non-steroidal anti-inflammatory drugs or glucocorticoids routinely to limit the inflammatory response.4,14 A prospective series that assessed the effect of antibiotics in the treatment of PIS showed no benefit of prolonged antibiotic prophylaxis beyond the usual period.15 We did not apply any specific treatment to the patients diagnosed with PIS. No patient was treated with anti-inflammatory drugs during the hospitalization, with exception for acetylsalicylic acid, which was prescribed to every patient.
ConclusionThe inflammatory syndrome after EVAR occurs in a significant percentage of patients, 21% in this study. The construction material of the stent-graft appears to be the key factor in the development of the inflammatory response. Polyester is the most important risk factor in the occurrence of the syndrome, in agreement with other published series.1,3,6,9,10 There was no PIS diagnosed in patients treated with ePTFE stents, and therefore, the occurrence of fever or leukocytosis on these patients should raise the suspicion of other complications. Although this inflammatory response might be exuberant, it was not associated with increased cardiovascular events in the postoperative period, and it is usually benign, well tolerated and self-limiting.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.