Carotid artery stenting (CAS) is a valid alternative to carotid endarterectomy with proper indications. In-stent restenosis (ISR) is a possible complication and there are multiple therapeutic options for severe ISR (>70%). The use of drug-eluting balloons (DEB) has increasing evidence as a new endovascular treatment for ISR. The authors report a case of recurrent ISR treated with a DEB.
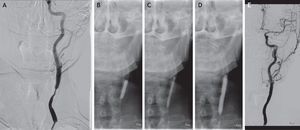
Case reportMale patient, 67 years-old, with a history of cervical radiation in 2006. In 2007, he had a stroke in the territory of the right internal carotid artery (ICA). The duplex ultrasound (DUS) showed right ICA occlusion and left ICA stenosis >70%. He underwent left CAS under filter protection, without complications. He was kept as an outpatient and in 2009 he presented ISR >70%. The patient was treated with re-stenting, without residual stenosis and had an uneventful course. In 2012, DUS revealed recurrent ISR >70%. Angioplasty with a paclitaxel-eluting balloon was performed, with distal cerebral protection, good imaging and hemodynamic results and an uneventful course. At 6 months of follow-up, the patient has no complications and no ISR documented by ultrasound.
ConclusionsThe use of DEB in the treatment of ISR after CAS is an emerging strategy with promising results.
O stenting carotídeo (CAS) é uma alternativa válida à endarteriectomia carotídea com indicações bem definidas. A restenose intra-stent (RIS) é uma complicação possível e são múltiplas as opções terapêuticas para o tratamento da restenose severa (>70%). O uso de drug-eluting balloons (DEB) tem evidência crescente como nova terapêutica endovascular em casos de RIS após CAS. Os autores descrevem um caso clínico de angioplastia com DEB por RIS recorrente.
Caso clínicoDoente de 67 anos, submetido a radioterapia cervical em 2006. Em 2007, apresentou AVC no território da artéria carótida interna (ACI) direita. O eco-Doppler demonstrou oclusão ACI direita e estenose ACI esquerda >70%. Foi submetido a CAS da ACI esquerda com protecção cerebral, sem complicações. Seguido em consulta externa e em 2009 o eco-Doppler revelou RIS >70%. Foi submetido a re-stenting, sem estenose residual e sem intercorrências. Em 2012, documentada por eco-Doppler recorrência de RIS >70%. Foi tratado com DEB, sob protecção cerebral, com bom resultado imagiológico e hemodinâmico e sem eventos neurológicos. O doente apresenta 6 meses de seguimento sem RIS demonstrada por eco-Doppler.
ConclusãoO uso de DEB no tratamento de RIS após CAS é uma estratégia emergente, com resultados promissores.
Carotid endarterectomy (CEA) is one of the most frequently performed vascular surgical procedures to prevent stroke associated with carotid stenosis in symptomatic and asymptomatic patients.1 It is the best-evaluated surgical procedure with an evidence-based medicine level of 1 and a recommendation level of A.2 Carotid artery stenting (CAS) was initially introduced as an alternative to CEA for high-risk patients or patients with hostile neck anatomy (status after radiation or previous cervical operations such as neck dissection or injury).1,2 The recent AHA/ASA guidelines recommend CAS for symptomatic >50% carotid artery stenosis and for several patient groups with asymptomatic carotid artery stenosis >70%.3
The clinical outcome of CAS is currently under investigation and equality of treatment, relative to CEA, has not yet been proven. Studies to date comparing the clinical outcome of CAS and CEA regarding stroke prevention, although large and randomized, have not shown a clear noninferiority of CAS to CEA.1 Randomised trials of patients with symptomatic carotid stenosis have shown that risk of periprocedural stroke is higher with stenting than with endarterectomy.4
Despite lack of level 1 evidence from randomized trials, the systematic use of cerebral protection appears to have reduced neurological complications during CAS, leading to consistently better outcomes even in “high surgical-risk” patients.5
The occurrence of in-stent restenosis (ISR) after CAS ranges from 3% to 20%, with half occurring within the first 6 months, over a relatively short (up to 2 years) follow-up.2,5–7
The etiology of ISR is still incompletely elucidated, but neointimal hyperplasia seems to play a key role in this process. Although constant proliferation of smooth muscle cells is responsible for deliberate and steady neointimal growth, in some cases ISR might have an abrupt course when associated with mural thrombus formation.2
The ideal treatment for carotid ISR is yet to be defined. Surgical and endovascular treatments have all been used, with variable results.6 We report the use of DEB in a patient who developed significant recurrent ISR after CAS.
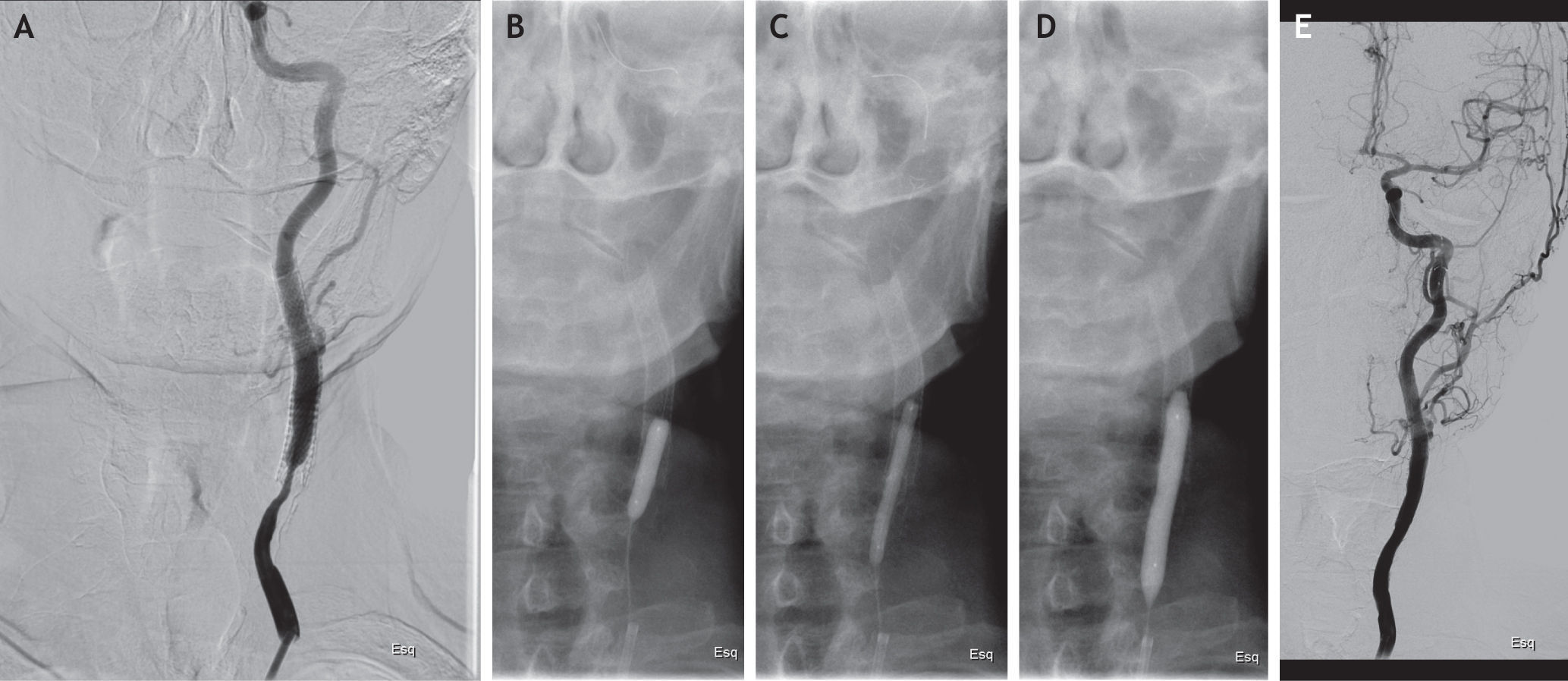
Case reportIn October 2007, a 62-year-old man was admitted to our department for severe asymptomatic stenosis (>70%) of the left internal carotid artery (ICA) and symptomatic occlusion of the right ICA, diagnosed by triplex scan. He was an ex-smoker and had a past medical history of dyslipidemia, hypertension and radiation therapy for larynx carcinoma in 2006. He underwent left CAS (Precise stent 7×40-mm, J&J Cordis) with cerebral protection without complications and with good immediate results. He was kept as an outpatient, under statin and dual antiplatelet therapy. In December 2009, routine ultrasound examination of the supra-aortic trunks had shown severe ISR (>70%). The patient was treated with re-stenting (two Precise stents of 8×30-mm + 7×30-mm, J&J Cordis), under filter protection, without residual stenosis and uneventful. In 2012, recurrent ISR >70% (peak systolic velocity – PSV=453cm/s) was detected at Doppler ultrasound follow-up examination. It was decided to perform a DEB angioplasty. The procedure was performed through right femoral approach, under local anesthesia. The left ICA was engaged in a telescopic fashion with a triple coaxial system formed by a 6-F sheath and a preloaded 4-F diagnostic catheter over a 0.035-inch glidewire. An intravenous heparin bolus (100U/kg) was given after sheath insertion. The 0.035-inch glidewire was exchanged for a 0.014-inch coronary wire and then the lesion was carefully crossed. Under distal embolic protection (Emboshield Abbott) the in-stent lesion was predilated using three peripheral artery balloons (Armada Abbott 3×40-mm + Armada Abbott 4×40-mm + Viatrac Abbott 5×20-mm). An over-the-wire paclitaxel-eluting balloon (In.Pact Pacific 6×40-mm, Medtronic Invatec) was then used. The balloon was inflated at 8 atmospheres for one minute and was well tolerated. After the procedure, there was no residual stenosis and no angiographic evidence of flow-limiting dissection or distal embolization (Fig. 1). The patient had an uneventful course and was discharged home by day two after DUS that revealed complete hemodynamic resolution of the stenosis (PSV=122cm/s).
Clinical and Doppler ultrasound follow-up was performed at 1 and 6 months and shows no related complications and no signs of ISR (PSV=128cm/s).
DiscussionThe occurrence of in-stent restenosis (ISR) after carotid artery stenting (CAS) ranges from 3% to 20% and varies with patient risk factors, lesion characteristics, and the location of the stent(s).2,5–7 However, the relatively wide range of ISR rates reported in previous studies may be the result of different definitions/diagnostic testing for ISR, patient selection bias, and length of follow-up.5
DUS is often used to monitor the patency of the stent and the occurrence of ISR. For routine evaluation of unstented carotid arteries, DUS is a well validated diagnostic test and the cut-off criteria for the different degrees of stenosis are clear.8,9 The peak systolic velocity (PSV) is the best predictor for the severity of the stenosis.10 However, the degree of restenosis in a stented carotid artery, measured according to the North American Symptomatic Carotid Endarterectomy Trial (NASCET) criteria on CTA or MRA, designed for native carotid arteries, could be overestimated. The presence of a stent increases flow velocities and the reason why this happens is not completely clear.1,7,10,11 A possible explanation might be pseudoacceleration of the detected velocity caused by the stent material interfering with the ultrasound signal.1 Another hypothesis has been proposed by Nederkoorn et al., who postulate elastic mismatch between stented and native areas of the artery.10 Possibly, blood flow and blood turbulence behave differently in an artificial stent than in a normal vessel and the presence of a stent can reduce compliance.7,10 Hakimi et al. reported that stent insertion into an ovine carotid artery without stenosis caused a 22% increase in the PSV and no change in the end-diastolic velocity (EDV). Distal do the in-stent stenosis, with moderate stenosis, stent insertion caused a 32% and 29% increases in the PSV and EDV, respectively; with severe stenosis, stent insertion caused a 49% and 56% increases in the PSV and EDV, respectively. They concluded by saying that stent insertion into an ovine carotid artery without stenosis caused a threefold compliance drop compared with that measured in the unstented artery.1
The use of an appropriate velocity threshold is central to the comparison of restenosis after carotid endarterectomy and carotid artery stenting.7 The 70% threshold to define high-grade restenosis is the most accepted threshold and has been used in the Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS), the Stent-Protected Angioplasty versus Carotid Endarterectomy (SPACE) trial, and the Endarterectomy versus Angioplasty in Patients with Severe Symptomatic Carotid Stenosis (EVA-3S) trial.12–14 The CREST definition of PSV ≥300cm/s to define >70% ISR was based on a comparison of more than 500 duplex ultrasound and anatomical comparative imaging studies.15 Several single-institution reports support the use of 300cm/s or more as an appropriate threshold to identify high-grade restenosis.1,7,10,16,17
While moderate ISR (50% to 69% by Doppler ultrasound or angiography) may be a relatively benign condition deserving “watchful waiting” in most cases, significant ISR (>70% stenosis) is usually considered an indication for revascularization.5 For a long time, it was thought that the symptomatology of carotid restenosis was benign.18 Indeed, progression to carotid occlusion and embolization and occurrence of ipsilateral transient ischaemic attack or stroke have been reported.5,7,11,12,15,18
Possible management options for carotid ISR include repeat balloon (or cutting balloon) angioplasty, re-stenting, surgical approach (CEA with stent removal, carotid artery bypass, or interposition graft) and brachytherapy.5,6,19 However, until now, there seems not to be a clear preferred treatment strategy for ISR and the therapeutic decision is taken on a case-by case basis.6,19 Balloon angioplasty and re-stenting can be performed with high acute success and low incidence of periprocedural complications, but rates of recurrent ISR, both early and (mainly) late, have been documented in up to 50% of the cases, with a small risk of cerebral embolization.5,6 This is a clear indicator of the need for new treatment strategies.
Recent evidence in the porcine internal carotid artery (ICA) model suggests that implantation of balloonexpandable drug-eluting stents (DES) may be associated with a significant reduction in in-stent neointimal hyperplasia compared to the bare metal stents.20 Tekieli et al. used the balloon-expandable zotarolimus-eluting stent to treat significant ISR after CAS in 7 patients. Despite acute favorable results, 1 patient developed symptomatic stent occlusion 1 month after the procedure, and another patient had a recurrent ISR at 12-month follow-up. In both cases, the stent protruding from the distal edge of the original stent was deformed and/or kinked.2 Evidence is accumulating to support the effectiveness of drug-eluting balloons (DEBs) as a new endovascular strategy for ISR treatment.5 While encouraging results have been reported in coronary arteries, limited data exist about the use of DEB to treat ISR in peripheral arteries.21–25 Indeed, only a few patients with ISR were enrolled in 2 randomized studies (22/154 in the THUNDER trial and 6/87 patients in the FemPac trial) comparing DEB vs. standard treatment, hampering conclusions about safety and efficacy.23,24 Vajda et al. recently reported DEB treatment of intracranial stent restenosis in 51 patients. Compared to conventional balloons, the ISR recurrence rate was significantly lower with DEB (9% vs. 50%) at 8-month follow-up, with a favorable clinical outcome.25 Specifically, there are no data on both safety and long-term results of DEB in patients with ISR after CAS. Montorsi et al. reported paclitaxel-eluting balloon treatment in 7 patients with ISR after CAS. Technical/procedural success was achieved in all cases and angiographic stenosis decreased from 83±5% to 18±6%. At 6 and 12 months, PSVs after DEB treatment were significantly lower compared to those assessed at comparable intervals after CAS and they had no ISR recurrence by Doppler ultrasound at a mean follow-up of 13.7 months. They concluded by saying that the significantly lower mean PSV after DEB compared to following stent implantation may indicate a substantial antiproliferative effect of the paclitaxel-coated balloon.5
Another concern is whether cerebral protection is really needed in patients undergoing endovascular treatment for ISR. Reimers et al. retrieved macroscopic debris in 71% of the examined filters in a large series of carotid ISR patients treated with angioplasty after CAS.26 Moreover, it has been shown that carotid ISR occurring after 24 to 36 months is often due to new atherosclerotic plaque formation rather than neointimal proliferation, increasing the embolization risk during balloon dilation to a level similar to that reported for CAS of de novo carotid artery stenosis.5,18 For these reasons, cerebral protection seems to be advisable when treating ISR.
Final technical issues refers to sizing of DEBs, duration of inflation and predilatation. A properly sized DEB plays an important role in achieving effective delivery of the antiproliferative drug within the vessel wall.5 The recommendations for DEB use in coronary ISR include predilation with a conventional balloon, DEB sizing according to a 0.8– 1:1 balloon-to-vessel ratio, and a single inflation at nominal pressure (8 atmospheres).5 Medtronic's In.Pact system has a natural coating that reduces the total drug elution time to 30 to 60 seconds; the majority of the drug is released within the first 30 seconds. The total drug load depends on both size and length of the balloon.6 DEBs might be useful mainly to treat diffuse, proliferative restenosis, while a potential use for predilation of long, calcified lesions (at high risk of stent fracture and restenosis) could be anticipated. Additionally, the possibility of routine predilation with DEB in the restenosis-prone intracranial circulation must be considered.6
ConclusionThere is increasing evidence for effectiveness of DEB angioplasty in the treatment of coronary and peripheral arteries, where the use of DEB (vs. conventional non-coated balloons) has been shown to reduce the restenosis rate.2 Data observed in other arterial beds was the rational for the application of DEB in the management of carotid ISR, that is emerging as a promising strategy with encouraging early and midterm results.5,6
Significant advantages of DEBs include fast, homogeneous release of a high dose of drug and absence of a permanent foreign body and polymeric material. Potential limitations of DEBs are the acute elastic recoil typical of balloon angioplasty and the variability of pharmacokinetics.6 Paclitaxel is the primary drug for DEB technology because of its potent antiproliferative effect and prolonged tissue retention.6
Further evidence in the form of randomized controlled trials is required to confirm whether DEBs are superior to other treatment options of post-CAS ISR.5