Background. To compare local injection of hemostatic agents and radiofrequency (RF)-assisted hemostasis in the management of bleeding from the portal vein with varying diameters and blood flow velocities.
Material and methods. Sixteen Bama pigs were used. Laparotomy was performed to expose the liver and inner diameters and blood flow velocities of the pre-injured portal vein in the hepatic segments and subsegments were measured. Vascular injuries in the portal vein were produced (4 in each pig). The pigs were randomly divided into two groups and local injection of hemostatic agents was performed in one group and RF-assisted hemostasis in the other, both techniques monitored by contrast-enhanced ultrasonogra-phy (CEUS). Time to hemostasis was measured, and the extent of liver injury was determined 2 h after treatment.
Results. In the local injection group, the rates of successful hemostasis were 100, 88.9, and 50% with portal veins with inner diameters of < 1 mm, 1-2 mm, and 2-3 mm, respectively, and the maximum time to achieve hemostasis was 24.0 ± 7.2 s. Hemostasis was not successful when the diameter was > 3 mm. In the RF-assisted group, hemostasis was successfully at all sites regardless of vessel diameter; however, the maximum time to achieve hemostasis was 156.8 ± 31.2 s. Injury to surrounding tissue was significantly greater in the RF-assisted group.
Conclusion. Both methods can achieve hemostasis with small diameter portal vein injuries; however, RF-assisted hemostasis is necessary for larger vessels, though it is associated with greater damage to surrounding tissue.
Liver injury is common in cases of abdominal trauma, and uncontrollable massive bleeding is the leading cause of death.1,2 The focus of the management of liver injury is to control the active bleeding while preserving as much of the hepatic parenchyma as possible.2,3 Conservative management may be applied when a patient is hemodynamically stable; however, control of active bleeding remains a complicated problem and segmental hepatectomy is necessary in some cases.2,4 When laparotomy is required, newly developed hemostatic dressings utilizing active clotting components are showing promise for reducing blood loss and the need for removal of hepatic tissue.5
Video-guided minimally invasive treatments of liver injury that utilize ultrasound, laparoscopy, and digital subtraction angiography (DSA) can control active bleeding, maximally preserve hepatic parenchyma, and decrease the possibility of re-injury, and have become the primary methods of treating liver injury.6–8 Contrast-enhanced ultrasonography (CEUS) can identify the location of liver injury and demonstrate active bleeding.6,9–11 Moreover, the ultrasound equipment is smaller, more convenient, and efficient compared to other imaging systems. CEUS-guided local injection of hemostatic agents and CEUS-guided radiofrequency-(RF)-assisted hemostasis are two newly developed minimal invasive hemostatic techniques. Previous studies have shown that the two methods result in satisfactory hemostatic effects on active bleeding inside the liver.6,12–16
In addition, RF-assisted precoagulation was shown to reduce blood loss when performed prior to laparoscopic liver resection.17 In preliminary research of CEUS-guided local injection of hemostatic agents, we have shown that this method is satisfactory for the treatment of grade I-III liver injuries.18–20
There are differences in the inner diameters and blood flow velocities of the vessels in hepatic segments and subsegments.21 Studies have shown that the management of active bleeding is more difficult when the ruptured vessels are closer to the porta hepatic.2 Thus, it is important to identify the characteristics of vessels for which local injection of hemostatic agents and RF hemostasis will be effective.
Thus, purpose of the study was to determine the effectiveness of local injection of hemostatic agents and RF-assisted hemostasis in the management of bleeding from the portal vein with varying diameters and blood flow velocities in a pig model.
Materials and MethodsExperimental animals and anesthesiaSixteen healthy Bama pigs, 8 females and 8 males, weighing 23.2 ± 1.8 kg were used in the current study. Intravenous anesthesia was performed with ketamine (6 mg/kg) and acetyl oxaprozin (0.05 mg/kg). Controlled ventilation was carried out with a ventilator during the experiments, anesthesia was maintained by a mixture of halothane and oxygen, and methadone was injected for pain control. An infusion of 0.9% saline at a rate of 10 ml/kg/h was given during the experiment, and blood pressure was kept stable by adjusting the infusion rate.22 This study was approved by the Institutional Review Board of our hospital, and all animals were treated according to established guidelines for the ethical care and treatment of laboratory animals.
EquipmentA Phillips CX 50 portable ultrasound machine (Phillips Healthcare, Andover, MA, USA) with an L12-5 high-frequency probe (12-5 MHz) was used. Gray-scale CEUS was used with low mechanical index gray-scale imaging, namely the contrast pulse sequencing (CPS) technique. The probe frequency at the time of imaging was 7.0 MHz, the mechanical index ranged from 0.16 to 0.18, and the focus for imaging was adjusted to the depth of the injury. The contrast agent used was SonoVue (5 mg/mL: Bracco, Italy), in which the microbubbles are filled with sulfur hexafluoride (SF6).
RF-assisted hemostasis was carried out with the Radionics Cool-tip™ RF Ablation System (Tyco Healthcare, USA), which includes the radiofrequency generator, therapeutic electrode, and cooling machine. The basic principle of the device is to stimulate plasma oscillations in tissue via RF energy. The ion-ion interactions produce heat, and the temperature can reach as high as 80-110 °C. The baseline power output is 80-100 W. A single-beam RF ablation needle was used in the present study; the length of the needle was 20 cm, and the length of the exposed end was 3 cm. RF-assisted hemostasis was performed as one phase with a total RF application time of 60-200 s, depending on the severity of the wound.
Selection of normal hepatic segments and subsegmentsAfter induction of anesthesia, laparotomy was performed to expose the liver. Two-dimensional (2D) gray-scale ultrasonography was carried out first to identify the portal vein in the hepatic segments and subsegments. The inner diameter of the portal vein was measured, and then blood flow velocities were measured by color Doppler and spectral Doppler sonography.
Establishment of injuriesIn each pig, 4 vascular injuries were produced in the portal vein of the hepatic segments and subsegments. Subsegment vascular injury was produced first, followed by hepatic segment vascular injury. For production of the injuries, 2D gray-scale ultra-sonography was used to identify the longitudinal axis of the vessel, and an 18 G aspiration needle (15 cm in length, 1.0 mm inner diameter, 1.2 mm outer diameter) was inserted through the vessels to be injured as a mark under ultrasound guidance. Then, the hepatic parenchyma was cut open with a scalpel along the aspiration needle to expose the vessels to be injured. The vessel was transected with a scalpel while avoiding injury to the hepatic artery (Figure 1). The aspiration needle was removed after the injury was made.
A. An aspiration needle was inserted to the portal vein in hepatic segments under two-dimensional gray-scale ultrasonography guidance. B. An aspiration needle was inserted to the portal vein in hepatic subsegment. C. Vessel transaction was carried out with a scalpel. Black arrow indicates that the needle was taken as a marker; white arrow indicates that the portal vein was transected with a scalpel.
Immediately after the portal vein injury was made, intravenous injection of the contrast agent (0.02 mL/kg) was administered. The ultrasound was switched to CPS mode, and the active bleeding from the ruptured vessel was observed. Establishment of the active bleeding model was confirmed when continuous bleeding from the incision could be identified by gross observation and bleeding could be identified by CEUS.
Local injection of hemostatic agentsImmediately after the portal vein injury model was confirmed, snake venom thrombin-like enzyme (Reptilase; Solco Basle Ltd., Switzerland) diluted to 2 mL was injected into the site of vascular injury with a needle under CEUS guidance. The injected dose was 1 Ku when the inner diameter of the vessel was < 2 mm, and it was 2 Ku when the inner diameter of the vessel was > 2 mm. After injection, α-cyanoacrylate adhesive (Medical Ear-Brain type EC Adhesive; Guangzhou Baiyun Medical Adhesive Co., Ltd.) was injected at the site of the damaged vessel. The injection dose was 1 mL when the inner diameter of the vessel was < 2 mm, and it was 2 mL when the inner diameter of the vessel was > 2 mm. The time to hemostasis was defined as the time from the completion of injection of α-cyanoacrylate to when no active bleeding was noted by gross observation and CEUS.
Cooled, single-beam RF-assisted hemostasisImmediately after the portal vein injury model was created, a RF radiofrequency electrode was inserted to the site of vascular injury under CEUS guidance. The RF needle was then held in place, and RF-assisted hemostasis was performed. The power of the RF generator was set to 80-100 W during the treatment, and the electrical resistance increased gradually after the RF-assisted hemostasis was begun. RF treatment was continued until the bleeding was noted to have stopped, at which time the needle was removed. The duration of treatment was as defined as the time from initiation of RF-assisted hemostasis to when gross observation and CEUS showed no active bleeding.
Treatment success and failureTreatment success was defined as cessation of active bleeding confirmed by gross observation and CEUS. Treatment failure was defined as continuous active bleeding observed by CEUS 5 min after treatment. At 2 h after the treatment, CEUS was performed to observe the extent of injury followed by laparotomy to observe the condition of tissues within the injury site and surrounding area. The injury was identified as the non-perfused region surrounded by a clear boundary. The boundary was traced manually at the maximal edge of the non-perfused region, and the extent of the injury was calculated automatically by the machine software.
Statistical analysesContinuous data were summarized as mean ± standard deviation and categorical data are presented as frequencies and percentage. Mixed models were used to compare the results from local injection of hemostatic agents and RF-assisted hemostasis, taking into consideration repeated measures. Data were analyzed using the SAS software version 9.0 (SAS Institute Inc., Cary, NC, USA). A value of p < 0.05 was considered to indicate statistical significance.
ResultsA total of 64 sites of portal vein injury were established in 16 pigs, which were divided randomly into two groups (each group had 32 sites), and each group was further divided into four subgroups according to the inner diameters of the injured vessels (Table 1). Gross, active bleeding was observed in all portal vein injuries (Figure 2A and 3A), and abnormal nodular or spurting increased uptake of contrast agent was observed by CEUS (Figure 2B and 3B). During the therapeutic process, hemodynamic instability occurred in 7 pigs which manifested as increased heart rate and slightly decreased blood pressure. The heart rate and the blood pressure returned to normal after successful hemostasis and fluid supplementation.
Blood flow velocities and hemostasis success rates.
| Blood flow velocity (cm/s) | Success rate, n (%) | |||||
|---|---|---|---|---|---|---|
| Inner diameter (mm) | Local injection of hemostatic agent | Radiofrequency assisted hemostasis | p | Local injection of hemostatic agents | Radiofrequency assisted hemostasis | p |
| n | n | |||||
| <1 | 7 (8.9 ± 2.1) | 6 (9.3 ± 2.2) | 0.7936 | 7 (100) | 6 (100) | - |
| 1-2 | 9 (12.0 ± 2.6) | 7 (11.9 ± 2.7) | 0.9338 | 8 (88.9) | 7 (100) | 1.000* |
| 2-3 | 10 (17.4 ± 4.2) | 10 (16.6 ± 3.8) | 0.6797 | 5 (50) | 10 (100) | 0.0330* |
| 3-4 | 6 (21.3 ± 4.1) | 9 (22.1 ± 3.6) | 0.5402 | 0 (0) | 9 (100) | <0.0001* |
A. Portal vein injuries in the hepatic subsegment. B. Contrast-enhanced ultrasonography (CEUS) revealed active bleeding from the injured vessel (arrows). C. Three minutes after local injection of hemostatic agent, CEUS shows no evidence of active bleeding, and the treated areas are in flaky irregular shape, or with no perfusion (arrows). D. Gross appearance of the liver after cessation of bleeding with local injection of hemostatic agent. Active bleeding stops, and the closure of the injury site is intact (arrows).
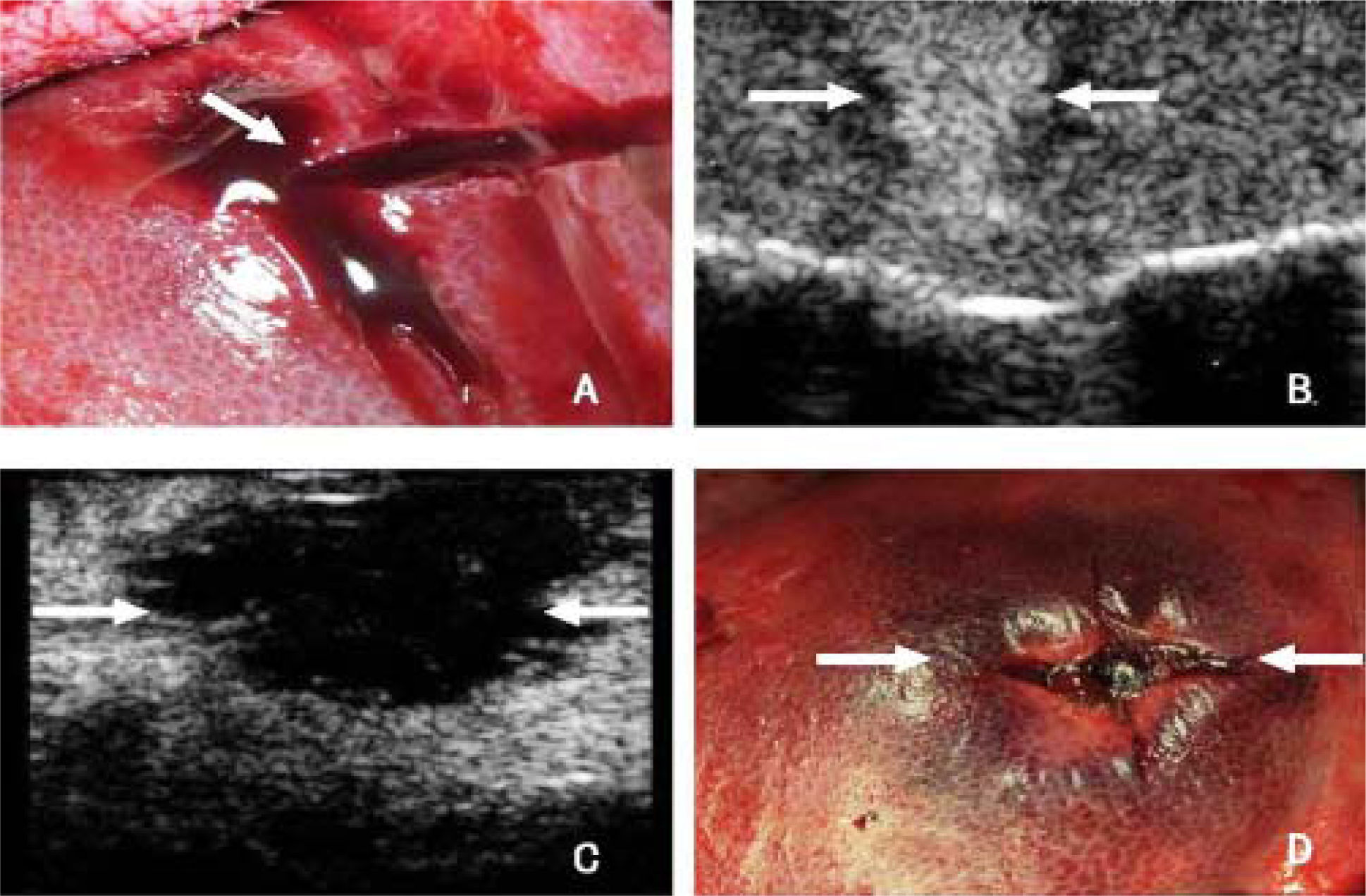
A. Portal vein injuries in the hepatic subsegment. B. Contrast-enhanced ultrasonography (CEUS) revealed active bleeding from the injured vessel (arrow). C. Three minutes after radiofrequency (RF)-assisted hemostasis, CEUS shows no evidence of active bleeding, and the treated areas present flaky nonperfusion zone with clear border (arrows). D. Gross appearance of the liver after RF-assisted hemostasis. Active bleeding stops, and the hepatic tissue in treated area is carbonized, darkened, and hardened (arrows).
Local injection of the hemostatic agents was administered to one group. After 5 min, CEUS revealed that active bleeding had stopped at all sites in the group with inner vessel diameters < 1 mm (Figure 2C), and all but one site in the group with vessel diameters between 1-2 mm. Active bleeding stopped in 50% (5/10) of sites in the group with 2-3 mm vessel diameters, and bleeding did not stop at any sites in the group with vessel diameter > 3 mm (Table 1). The time to successful hemostasis and the extent of the injured area 2 h after treatment as determined by CEUS are shown in table 2. After successful hemostasis, blood clots and a solid adhesive film were observed on the surface of the liver lesion, the wound was sealed, and bleeding had stopped (Figure 2D). When hemostasis failed, active bleeding continued and the thrombin and hemostatic adhesive were washed out from the bleeding wound and adhered to the surface of the liver.
Time to successful hemostasis and extent of injury.
| Time to successful hemostasis (s) | Extent of injury (cm2) | |||||
|---|---|---|---|---|---|---|
| Inner diameter (mm) | Local injection of hemostatic agent | Radiofrequency assisted hemostasis | p | Local injection of hemostatic agents | Radiofrequency assisted hemostasis | p |
| n | n | |||||
| <1 | 7 (16.7 ± 2.0) | 6 (97.5 ± 16.2) | < 0.0001* | 6.6 ± 2.0 | 9.6 ± 1.2 | 0.0129* |
| 1-2 | 8 (23.0 ± 3.9) | 7 (110.4 ± 24.0) | 0.0005* | 6.9 ± 1.6 | 11.7 ± 2.5 | 0.0066* |
| 2-3 | 5 (24.0 ± 7.2) | 10 (156.8 ± 31.2) | 0.0008* | 7.2 ± 1.3 | 13.2 ± 2.3 | 0.0015* |
| 3-4 | 0 (-) | 9 (154.3 ± 38.6) | < 0.0001* | - | 13.9 ± 2.1 | - |
RF-assisted hemostasis was applied in the other group. Five minutes after treatment, CEUS revealed that bleeding had stopped in all treated vessels in all subgroups (Table 1 and Figure 3C). The time to successful hemostasis and the extent of the injured area measured 2 h after the treatment as determined by CEUS are shown table 2. After successful hemostasis, complete solidification occurred inside the liver at the site of the injury and its surface, and carbo-nation and shrinkage were noted in some liver tissue (Figure 3D).
The time to successful hemostasis was greater in the RF group as compared to the hemostatic agents group, whereas, the extent of liver injury was greater in the RF group as compared to the hemostatic agent group (both, p < 0.05) (Table 2). When the vessel diameter was > 2 mm, the success rate of RF-assisted hemostasis was significantly greater than that of local injection of hemostatic agents (p < 0.05).
DiscussionIn this study of RF-assisted hemostasis and local injection of hemostatic agents for the treatment of portal vein injury in a pig model, both methods achieved hemostasis when the diameter of the injured vessel < 2 mm, however, the success rate of RF-assisted hemostasis was significantly greater when the diameter of the injured vessel was > 2 mm. However, RF-assisted hemostasis produced much greater damage to the liver parenchyma than did the local injection of hemostatic agents. Though uncommon, damage caused by hemostatic agents may include injury to tissue from the needle puncture or a local inflammatory response to the hemostatic materials.
Liver injuries are most commonly classified with American Association for the Surgery of Trauma (AAST) grading system.23 In this system, grade I-IV liver injuries are generally classified according to the depth of the liver laceration and the extent of hematoma, and active bleeding is not a major factor in the classification. However, active bleeding may also occur in grade II-IV liver injuries, especially in hepatic segments and subsegments with relatively thin hepatic parenchyma where vascular rupture can occur in branches of major vessels resulting in hemodynamic instability.2,24 In patients who are he-modynamically unstable, surgery it typically required; however, the surgical procedure itself is complicated, causes further injury to the patients, and is associated with significant postoperative complications. Thus, there is great interest in minimally invasive treatments for patients with liver injuries associated with hemodynamic instability.
In this study, the hemostatic agents Reptilase and α-cyanoacrylate adhesive were injected into the injured portal veins under CEUS guidance. Reptilase, a snake venom thrombin-like enzyme has a thrombin-like action, and can rapidly form blood clots making it useful as a topical hemostatic agent to stop blee-ding.25 However, the blood clots produced by it are relatively soft and have a low tensile strength, and thus cannot completely control rapid bleeding from large wounds. On the contrary, α-cyanoacrylate adhesive quickly solidifies in an environment containing tissue fluid and blood, and thus can function as a hemostat and wound plug even if there is rapid bleeding.26 Other studies have reported good results with the use of hemocoagulase atrox, another snake venom thrombin-like enzyme, with α-cyanoacrylate. Tang, et al.6 studied atrox and cyanoacrylate in a canine model of blunt hepatic trauma, and found that transcutaneous injection reduced blood loss and stopped bleeding compared to a control group. Lv, et al.15 reported similar results in a canine model in which a 6.0 x 4.5 cm hepatic wound was created via laparotomy. Li, et al.16 also reported good result for the injection of hemostatic agents to control bleeding in a canine model of blunt splenic trauma, and laparotomy 3 weeks after injection showed that the injury sites had completely healed.
Our previous studies have proven the effectiveness of local injection of hemostatic agents in controlling active bleeding in liver wounds due to blunt trauma.18–20 However, in those studies we did not examine the effectiveness of local injection when direct injury to vessels was present, or for which size of vessels it would be effective on. The present study showed that this method was very effective in controlling active bleeding after the hepatic segment and subsegment portal vein injury when the inner diameter of the portal vein inside the injury site was < 2 mm. However, this method was not ideal for the vessels when the inner diameter of the portal vein inside the injury site was > 2 mm. When the inner diameter of the portal vein inside the injury site was > 3 mm, this method was ineffective at stopping bleeding. To determine the possible reasons, we directly observed the related vessels during treatment. When the inner diameter of the ruptured vessel was relatively large and the blood flow velocity relatively fast, the injected hemostatic agents were washed out by the rapidly flowing blood from the vascular stump, and the success rate of hemostasis decreased. On the contrary, when the inner diameter of the ruptured vessel was relatively small and the blood flow velocity relatively slow, the injected hemostatic agents were more likely to adhere to the vascular stump and the hemostatic outcome was satisfactory.
Ultrasound-guided percutaneous RF ablation (RFA) has become an important nonsurgical treatment for liver cancer, and satisfactory clinical outcomes have been achieved.27,28 RFA induces coagulation and necrosis of tumors by increasing the local tissue temperature. The use of RF to stop bleeding in the liver is still in the experimental stage; however, preliminary results have indicated that this method can effectively stop active bleeding in grade II-IV liver injuries.13 Zacharoulis, et al.14 studied the use of a bipolar RF device to control hepatic and splenic bleeding from stab wounds in a pig model. Bleeding was controlled in central and peripheral lesions, and no immediate complications were noted up to 6 weeks after the procedure when the animals were killed for autopsy examination. The authors, however, did not mention the exact diameter of the vessels in which the bleeding could be controlled. Yekou, et al.12 used a multipolar RF device to control bleeding in a pig model of blunt hepatic trauma, and found that hemostasis was achieved in a mean of 8.5 ± 4.5 min of RF treatment in all pigs.
The hemostatic mechanism of RF-assisted is thermal coagulation of the bleeding vessel and surrounding tissue. The results of this study showed that RF-assisted hemostasis is effective for vessels up to a diameter of 4 mm. This greater effective range is likely because thermal coagulation is less affected by blood flow than the application of hemostatic agents, i.e., the hemostatic agents are not washed away by a low blood flow.
During the present study, we found that the time to hemostasis was greater with RF-assisted hemostasis, and greater damage to surrounding liver tissue was observed than with the injection of hemos-tatic agents. A large amount of damaged liver parenchyma may result in liver failure,29 and the larger effective area of RF has the potential to damage other vessels, the biliary system, and adjacent organs such as the bowel or diaphragm.30 Local injection of hemostatic agents only results in the formation of local blood clots without damaging adjacent areas of the liver.
The results of this study suggest that both methods have the potential for clinical application. When the inner diameter of the injured portal vein is < 2 mm, local injection of hemostatic agents can provide hemostasis and maximally preserve the hepatic parenchyma with a relatively low risk of complications. When the inner diameter of the injured portal vein is 2-4 mm, RF-assisted hemostasis is effective for controlling bleeding but results in greater injury to hepatic parenchyma.
There are limitations of the study which should be considered. The present study only investigated the effectiveness of the two methods in the treatment of active bleeding from the portal vein; active bleeding from hepatic veins was not investigated and should be studied further. Furthermore, mid-and long-term outcomes and complications of the two methods have not yet been investigated. So the feasibilities of their clinical applications should be confirmed by further studies.
ConclusionIn summary, both local injection of hemostatic agents and RF-assisted hemostasis can achieve he-mostasis in liver injuries associated with small vessels. For injuries to larger vessels, RF-assisted hemostasis is necessary, though it is associated with greater damage to surrounding tissue. CEUS is a useful tool for evaluating hepatic injury and bleeding.
AcknowledegmentThe financial support of the Beijing Municipal Science & Technology Commission Programs (No. Z101107050210046) are gratefully acknowledged.