Chronological aging is characterized by a persistent decline in age-specific fitness components due to internal physiological degeneration [1]. In addition, a hallmark feature of organismal ageing is the inability of the organs to maintain their homeostasis and respond poorly to the physiological stress and external insults [2]. While the term ageing is usually associated with organs and organism, the process at cellular level is termed senescence which is a permanent cell cycle arrest. At the cellular level, senescence, a permanent cell cycle arrest, manifests through two primary mechanisms: replicative senescence, attributed to telomere erosion, and stress-induced premature senescence, accelerated by oxidative DNA damage or oncogene mediation [3]. Aged organs exhibit degenerative changes such as loss of cellularity, extracellular matrix deposition, diminished regenerative capacity, and the presence of senescent cells.
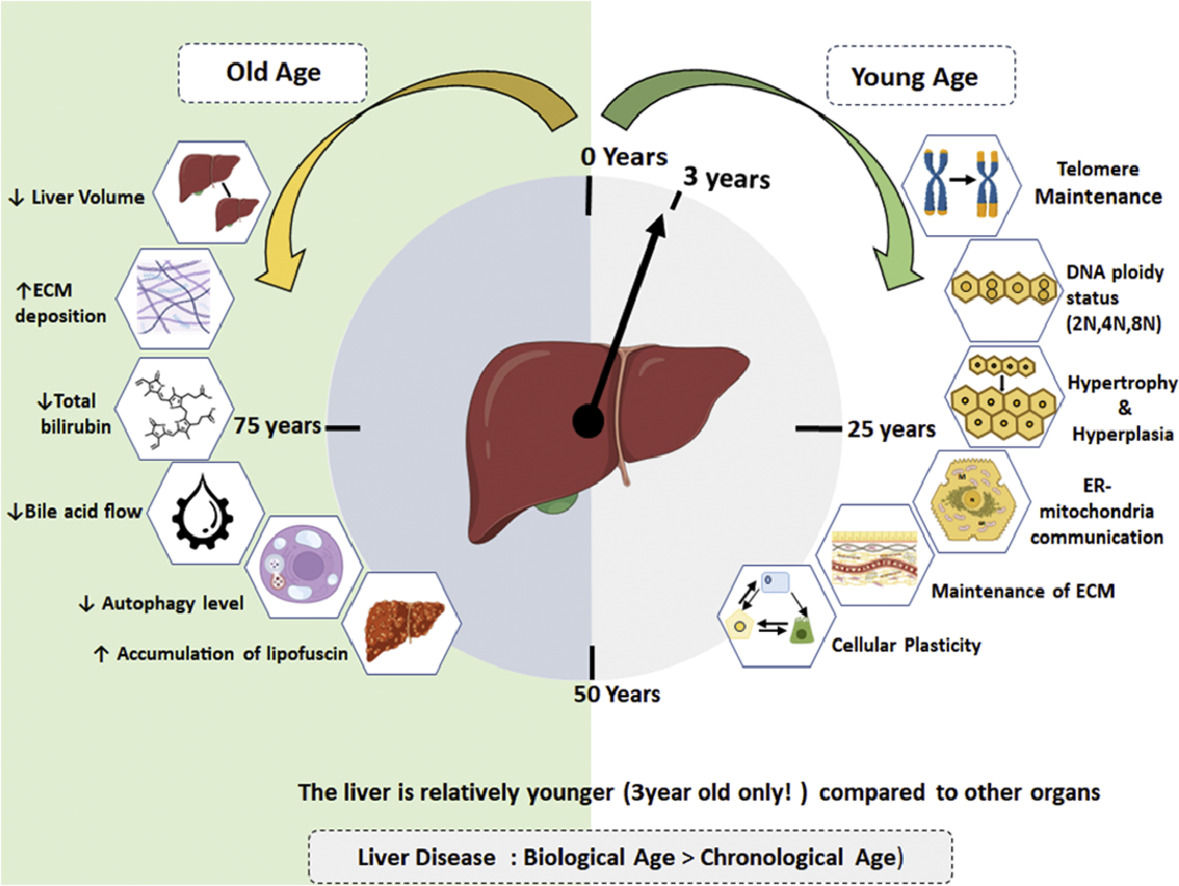
Notably, organs vary in their ability to maintain homeostasis with age [4]. The liver, despite undergoing morphological and physiological alterations, maintains substantial function, with liver function tests remaining nearly normal [5]. The perception is that the liver is resilient to aging, earning the phrase "liver does not care about age" [6,7]. In general aging liver experiences morphological changes, including darkening due to lipofuscin accumulation, volume loss, pseudo-capillarization, reduced blood flow, and fibrosis.
Studies by Douglas Schumaker comparing young and aged groups (60-90 years) revealed a decline in total bilirubin and an increase in cholesterol levels with age [5]. Geriatric patients exhibit reduced bile acid synthesis and flow, increasing susceptibility to gallbladder stones [8]. Those above 70 years show a decline in liver-specific cytochrome P450, affecting drug clearance and predisposing them to adverse drug reactions [9] Although a modest decrease in albumin concentrations is noted, the impact on ischemia-modified albumin levels in the elderly remains unclear [10]. Hepatocytes undergo changes with chronological aging, including the accumulation of oxidized proteins, decreased endoplasmic reticulum and autophagy levels, nuclear volume alterations, and vacuolation [11] (Fig. 1).
The aged hepatocytes both in rodents and humans, generally exhibits a decline in regenerative capacity due to hypoacetylated chromatin [12]. In liver transplant settings, the policy is typically to avoid donors above 50 years. However, some studies report favourable outcomes with livers from septuagenarian, octogenarian, and very old donors under special circumstances [13,14]. A rodent model study suggests that aged livers preserve regenerative potential under specific conditions, response albeit somewhat weaker and slower [15]. Additionally, it has been noted that elderly hepatocyte progenitor cells can effectively repopulate the liver following injury, indicating that they retain their regenerative potential [12].
In an animal model of liver transplant, a study demonstrated the sustained functionality of orthotopic liver grafts with advancing age, despite age-related histological features like fibrosis and pigment deposition [16]. These findings not only have clinical implications for liver transplant surgery but also highlight the intriguing ability of aging livers to cope well during regeneration.
A recent study has indicated that during liver regeneration the chromatin landscape changes towards a more “younger-pattern”. The authors showed that ageing is associated with global increase of hetrochromatization marks H3K27me3, at special chromatin sites “age-domains” which declined significantly following regeneration. Interestingly, the chromatin and transcriptional profiles of regenerated older livers became more like those of younger livers indicating the remarkable property of reversing the ageing [17]
In conditions of severe acute liver failure, the liver regains back its microarchitecture by activating a subset of ANXA2+migratory hepatocytes which move as a sheet to close the necrotic wound area. The necrotic wound closure precedes hepatocyte proliferation and appears similar to skin wound healing so as to maintain the gut-liver barrier [18]
Routine liver function tests, such as alkaline phosphatase and transaminase activities, remain unaffected by age, suggesting minimal hepatocyte degeneration [19]. In the absence of good markers to detect cellular senescence in in vivo tissues, it is difficult to assess the chronological ageing in liver. Regardless of this fact, it is remarkable that despite aging, liver can maintain most of its regular functions, albeit with subtle changes, without incurring any pathological consequences. [20] When compared to other major organs like the heart, kidney, and brain, the liver exhibits a relatively slower decline in function. This is possibly due to an inherent compensatory mechanism or a hyperfunctioning state [19-22].
The current review predominantly delves into the distinctive biological aspects of the hepatocyte maintaining the organ function throughout normal aging, with a brief discussion on the impact of aging on end-stage liver disease.
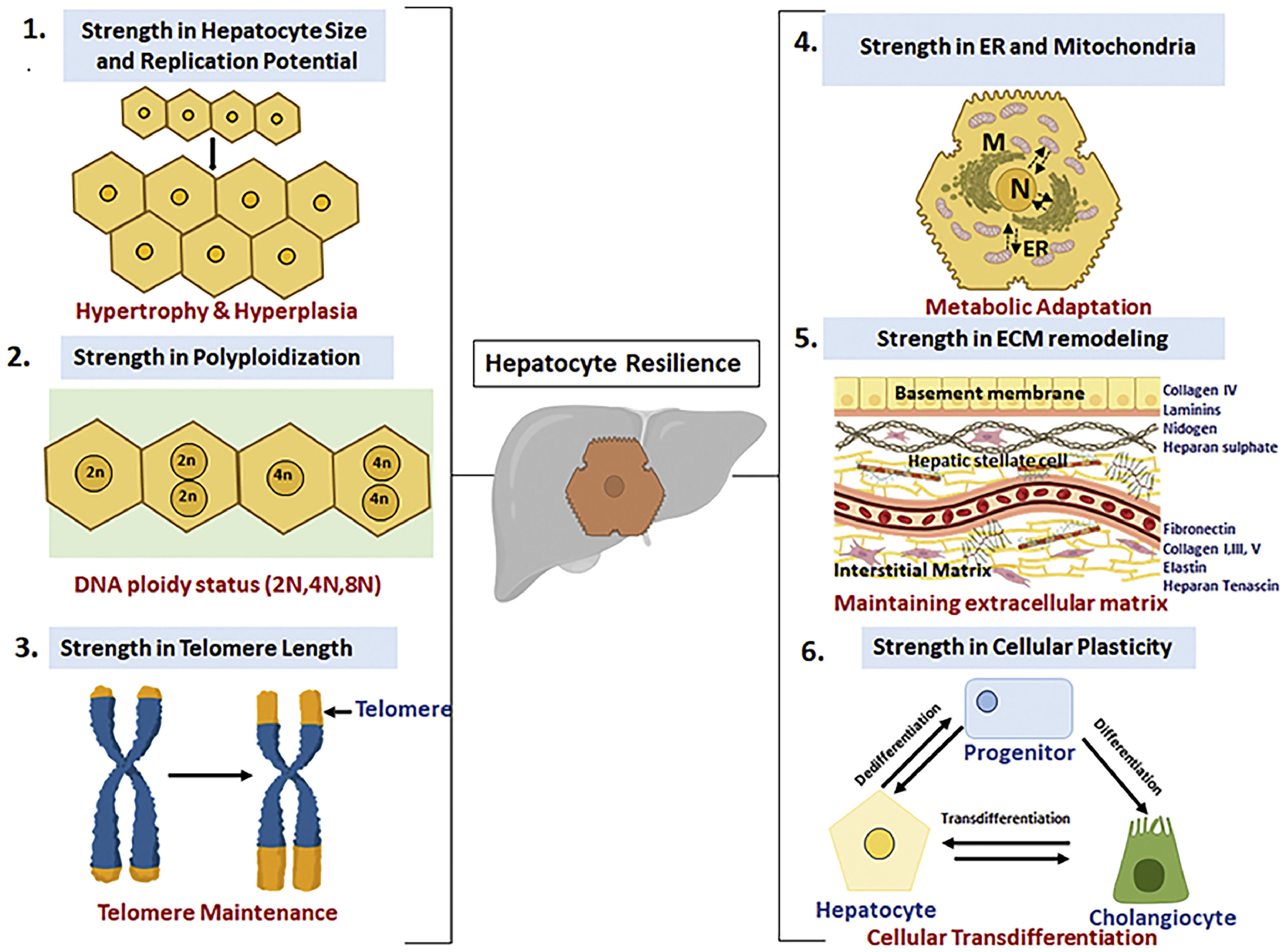
2Compensatory mechanisms for normalizing liver function with advancing agingHow, does liver relatively safeguards its functions with advancing age? An obvious answer is its large cellular reserve, viz. the hepatocytes which constitutes about 80-90% of the total cell population and their regenerative potential. However, there are additional important cellular aspects about hepatocytes which helps in maintaining the cellular homeostasis and these include: (a) hypertrophy or increase in hepatocyte size in absence of cell division, (b) changes in the ploidy status of hepatocyte also referred as “ploidy conveyor”, (c) preservation of telomere length thereby making it “youthful”, (d) remodelling and maintenance of the extracellular matrix and microenvironment with advancing ageing and (e) cellular plasticity of cell to transdifferentiate (Figure 2). These unique properties of the liver may contribute in the “youthfulness’ of the liver and are elaborated in detail below.
Adaptive strategies utilized by hepatocytes for resilience in the face of chronological aging: (1) Cellular hypertrophy in conditions of increased demand, (2) Ploidy conveyor and ploidy reversal, (3) Increased telomere length of hepatocytes, (4) Inter-organellar stress response mechanism for meatbolic adaptation, (5) remodelling of extracellular matrix for homeostasis and (6) Cellular plasticity and memory during regeneration.
A classic feature of liver with advancing age is increase in hepatocyte size even with decline in liver volume and number of hepatocytes [5,23]. The increase in cell size or hypertrophy, with age, is interpreted as a balancing act of liver to cope with its metabolic functioning in event of declining cell number. In fact, the importance of cellular hypertrophy in context of organ homeostasis has come from studies on liver regeneration in rodent model. Very little is known especially with regard to histomorphological features of hepatocytes from human liver following hepatectomy. For long it was perceived that hyperplasia or increase in hepatocyte number by cellular division is the first line of action during liver injury leading to regeneration. However, later evidence has clearly pointed that gain in liver mass, at least in animal models, includes both cellular hypertrophic and hyperplastic changes. In rodent models, with a small loss of liver volume such as 30% partial hepatectomy, the preferred way to gain liver function is by hypertrophy in absence of cell division. However, in case of loss of large liver volume, such as 70% hepatectomy, both hypertrophy and hyperplasia contribute equally to gain in liver volume [23]. With advancing age, the liver volume declines by 30% and in absence of hyperplasia, the increase in hepatocyte size by hypertrophic mode may serve as a compensatory mechanism to improve the liver function [24]. Indeed, it has been recently noted that in human liver, the hepatocytes from older individuals (55-65years) are much larger in size compared to the young adults (21-30years). While the authors also noted increased SA-betagalactosidase positivity in the older hepatocytes indicative of senescence, it is also possible that increased cell size is actually indicative of a compensatory state to cope with liver ageing [25]. Thus, we surmise that hypertrophy might precede the proliferative phase as a compensatory mechanism in case of atrophy of hepatocytes during normal ageing.
What is the mechanism by which the increase in hepatocyte cell size occurs? Evidence from animal models of liver regeneration especially in the pregnant mice has suggested a role of AKT-mTOR pathway in the hypertrophic growth of the hepatocytes. Intriguingly, it was observed that liver volume restoration after two days of partial hepatectomy was 96% in aged pregnant mice and only 46% in non-pregnant aged mice [26]. Blocking the AKT/mTOR pathway with rapamycin prevented the hypertrophic response in pregnant aged mice. Conversely, bisperoxovanadium 1,10-phenanthroline [bpV(phen)], an AKT activator could turn on hypertrophic response and increase the regenerative potential of aged non-pregnant mice. Why is hypertrophy preferred over hyperplasia? Though the exact reason is not clear, it is possible that during normal physiological process of ageing an increase in cell size by hypertrophy is a more economical, easy and safe mechanism for compensating the liver function. In contrast, the hyperplastic mode of increasing cell number would be not only more energy consuming, but would also increase the risk of neoplasia if it is not regulated appropriately. Thus, it is suggested that at least during regeneration in aged liver the hypertrophic regeneration module is the preferred over hyperplasia as it is failsafe mechanism and less affected by aging [27]. Though this is beyond the scope of this article, it is a noteworthy paradox that while studies on mice prove that pregnancy improves the regenerative capacity of liver; in human, hepatitis E virus infection during pregnancy leads to fulminant hepatic failure [28]. Further studies are needed in human to prove the beneficial effects of hyperplasia in both normal ageing and during organ regeneration.
2.2Genetic variation due to change in ploidy status for coping with stressYet another specific age-related hepatic change is the increase in number of binucleated cell and reduction in mitochondrial number [29-30]. In fact, liver samples taken from elderly individuals as well as aged rodent models show an increase in polyploidy status and binuclear index [31-33]. However, there are some conflicting reports where no change in ploidy status has also been reported in aged liver samples [34-35].
Does the increase in binucleate status of aged liver provide any advantage to the ageing liver? The term “ploidy conveyor” was coined to describe the state of hepatocyte polyploidization, ploidy reversal and aneuploidy [36]. Although the exact mechanism of how polyploidy confers advantage to hepatocytes is still not clear, it has been suggested that ploidy conveyor protects the hepatocytes under stressful conditions especially during xenobiotic exposure or nutritional injury. During ageing, the presence of extra genetic material possibly helps the hepatocytes to function to its full capacity and also help endure the age-related stress factors. In this regard, it has been noted that polyploidization mitigates age-associated accumulation of lipid and helps preserve the homeostasis [37]. Also, polyploid hepatocytes may select for additional copies of wild type gene allele so as to counteract the mutant alleles in ageing liver. Polyploid hepatocytes may help in turnover of hepatocytes as the organism ages for maintaining organ functioning [38].
On the downside, it has to be also noted that polyploidy and aneuploidy are hallmark features of cancer and indeed with increasing age there is an increase in incidence of hepatocellular carcinoma.
2.3Cellular rejuvenation by maintenance of telomere length by hepatocytesTelomeres are the protective ends of the chromosomes which continue to shorten with each round of cell division until they reach a critical short length. At this stage, the cells undergo a crisis state and enter into a permanent state of growth arrest or replicative senescence. Telomeres are cellular biological clock which determines whether the cell will continue to divide or ultimately stop dividing. Progressive loss of telomere has been seen in both human and rodent liver during the normal process of ageing [39-41]. In all these studies the total liver cells were pooled to calculate the telomere attrition with age and on an average the telomere reduction rate of 29-60 base pair (bp) per year was reported in the liver [42]. Liver is composed of many cell types of which 60-80% of cells are hepatocytes and rest consist of immune cells, stellate cells, cholangiocytes, Kupffer cells etc [43]. It has to be noted that telomere length disparity occurs not only between different tissue types but also different cell types [44-46]. In this regard a remarkable study by Verma et al has shown that different cell types in the liver age at different rates. Unlike the previous reports, this work clearly showed that both cholangiocytes and hepatocytes sustained their telomere length with advancing age. In contrast, hepatic stellate cells, Kupffer cells and endothelial sinusoidal cells showed telomere attrition with age. This study thus indicated that different hepatic cell lineages age at different rates [47]. Why should hepatocytes and cholangiocytes maintain their telomere length unlike the other cell types? One argument provided by the authors is, the “low turnover of these cells and thereby preserving their regenerative capacity”. It is also possible that unlike other cells, hepatocytes and cholangiocytes have higher telomerase activity which in turn can help maintain the telomere length with advancing age. If decline in telomere length is indicator of age and hepatocyte sustain their telomere length, then one can argue that even with the chronological ageing the liver is considerably young compared to other organs and hence has a good regenerative capacity. Having said that, one cannot simply ignore the numerous reports which indicate a decline in regenerative capacity of aged liver. Oxidative stress due to excessive reactive oxygen species is another cause of permanent growth arrest in cells and is not associated with telomere shortening. This is usually called Stress Induced Premature Senescence (SIPS). Thus, besides the telomere attrition, balance between the redox status and oxidative stress must be ultimately determining the youthfulness of liver cells.
While senescent cells are considered an irreversible arrest state, a very recent study has suggested that senescence is reversible as senescent cells from both human and also aged mice reverts to original proliferative state following serial transplantation [48]. Further the authors noted hepatocytes showed no telomere shortening, instead they showed activation of telomerase which can not only help prevent telomere attrition but also help bypass the barrier of senescence. Further studies are needed to show the exact mechanism by which senescence can be bypassed. It is worth noting here that a combination of “six-factor gene cocktail” could efficiently reverse and rejuvenate the senescent fibroblasts derived from centenarian individual by cellular reprogramming [49]. Chromatin reprogramming in regenerating liver sets the clock back to a more youthful state. The chromatin and transcriptional landscapes of regenerated aged livers showed a shift towards those found in younger livers, highlighting a notable ability to reverse aging [17]. Thus, it needs to be seen if hepatocytes can retain their “youthfulness” by chromatin reprogramming and reset their cellular physiology to function normally during chronological ageing.
2.4Maintaining extracellular matrix for cellular functioningBesides the genetic, epigenetic and signalling networks that governs the process of senescence, yet another crucial factor influencing cellular ageing is the microenvironment and extracellular matrix where the cell resides in the organ. In fact, it has been noted that extracellular matrix from young cells can efficiently rejuvenate and restore the proliferative capacity of the senescent cells [50]. Similar to this study, it has been noted that hepatocytes from cirrhotic rat liver which showed signs of replicative senescence and apoptosis not only engrafted normally in a noncirrhotic microenvironment, but also recovered their compromised functions [51]. These studies thus clearly indicate that microenvironment is critical for normal cellular homeostasis. To maintain healthy status, tissue remodelling of extracellular matrix is important for repair or removal of damaged protein and deposition of new protein [52]. Thus, we surmise that healthy ageing of liver is possible if liver is able to maintain a conducive microenvironment by retaining the remodelling the extracellular matrix by a balancing act of “repair-replacement” without any overt disbalance. However, it has to be noted that moderate fibrosis does occur with normal ageing with deposition of collagen type- III mainly in the periportal compartment which in turn is due to reduced activity of metallo-matrix proteinase (MMP) [53]. The impact of moderate fibrosis on functioning of hepatocytes is still not clearly understood, however it is worth noting that functioning of liver is fairly normal and well compensated even in fibrotic patients. In fact, patients of liver disease with less severe fibrosis remain stable [54]
2.5Mitochondrial and endoplasmic reticulum based adaptations as resilience for stress adaptation and metabolism as a survival for organ tolerance in normal ageingMitochondria and ER are particularly critical in liver compared to other tissues as hepatocytes are responsible for metabolic and detoxification functions. The hepatocytes possess a remarkable ability to adjust to fluctuations in nutrient supply and hence hepatocytes are a highly specialized cell with abundant mitochondria and expansive ER network, The ER-mitochondria contact points, referred to as mitochondrial-associated membranes (MAMs) is a hallmark feature of hepatocytes helping in metabolic fluctuations. Hepatic subcellular organization of the ER architecture are highly dynamic, integrated with the metabolic state and critical for adaptive homeostasis and tissue health [55]
Hepatocyte house between 1000 and 2000 mitochondrion to meet the metabolic demand of the organ. Decrease in mitochondria together with its increase in volume has been noted in ageing rodent and human liver [56]. Additionally, a decrease in respiratory chain activity together with decline in mitochondrial DNA and protein levels have been also noted in older rodent models [57]. While these older studies concluded the declining activity of mitochondria is linked to process of ageing, recent studies refute this popular idea. In fact, the new theory called “rate of living” proposes that inhibition of mitochondrial respiration favours slow metabolism which in turn slows down the process of ageing [58]. In fact, as per the new proposition inhibition of mitochondria respiration declines aging by either increasing the AMPK signalling or the ROS levels. A decrease in mitochondrial respiration will lead to increase in the AMP:ATP ratio, which in turn is sensed by the AMPK, which is now identified as a lifespan regulator at least in lower model organisms. In fact, activation of AMPK turns on autophagy which is basically a survival strategy under stress [59]. It has been shown that activation of chaperone mediated autophagy prevents accumulation of damaged proteins which in turn helps in improving hepatic function with ageing [60].
Recent study has shown that mitochondria in liver cells can adapt very fast for energy metabolism in response to food. Rapid mitochondrial fragmentation occurs in liver in response to sensory food perception which in turn triggers insulin mediated glucose production. The mitochondrial fragmentation helps in insulin sensitivity following food intake [61].
Reactive Oxygen Species (ROS) generated in mitochondria was always considered a toxic by product of aerobic metabolism that leads to oxidative stress and damage to cellular macromolecules such as nucleic acids, proteins and lipids thereby mediating the process of ageing. Contrary to this popular belief, it has now emerged that ROS functions as key mediator in the signal transduction cascade [62-63]. ROS has been shown to be key mediator of retrograde signalling from mitochondria to nucleus that increases the activity of hypoxia inducible factor which in turn regulates lifespan [64].
The turning on of the Unfolded Protein Response (UPR) Mitochondrial Responses (UPRMT) is fairly a new and emerging area that helps in quality control and optimal functioning of the mitochondria. It appears as yet another survival strategy protecting liver from accruing damage when fed on high fat diet [65]. While the role of UPRMT response in longevity is well documented in C. elegans, its role in mammalian longevity is still elusive. A recent study shows a link between UPRMT and stem cell rejuvenation [66]. And we recently showed that overexpressing mitochondrial protein, ClpP, a key component of UPRMT can ameliorate senescence response [67].
2.6Cellular Plasticity and Cellular MemoryA remarkable property of parenchymal compartment of liver is the plasticity of cells whereby the cholangiocytes can transdifferentiate to hepatocytes and vice versa [68-69].
In conditions of chronic injury when hepatocytes either undergo cell death or even premature ageing /senescence, it has been noted that biliary epithelial cells (BECs) can potentially give rise to new hepatocytes with bipotent phenotype (HNF/CK19+) in the periportal region [70]. It has been noted that cellular plasticity can decline with chronological ageing due to increase in levels of histone hypoacetylation. However, inhibiting deacetylases could revive the compromised cellular plasticity The reprogramming of the cells indicates that hepatocytes and cholangiocytes are equipped with active chromatin reprogramming [12].
It has been shown the hepatocytes taken from elderly individuals have declined cellular plasticity, however treatment with HDAC inhibitors improved both the regenerative capacity as well as plasticity of aged hepatocytes [12]. This finding suggests that elderly hepatocytes too can be rejuvenated with age reversal, moreover the progenitors cells taken from elderly individuals retain their ability to proliferate and repopulate injured liver in animal model.
3Role of premature senescence in chronic liver diseaseWhile the preceding write-up discussed the compensatory mechanisms helping in maintain liver functions with chronological ageing, it is equally important to note senescence associated changes accompany liver disease pathology. A developing field in the area of ageing is “gerosciences” which is gaining much spotlight and attention because of the close association between process of aging and related chronic disease [71]. In fact, end stage liver disease, irrespective of the etiology, shows pathology of senescence changes in different cell types which in turn correlated with poor clinical outcome [72]. Thus, it is hypothesized that premature ageing is a causative factor in the genesis and organ failure in chronic liver disease. Briefly, the chronic liver disease is associated with four distinct features which are reminiscent of onset of premature ageing and these include (a) inflammaging, which is low-grade chronic inflammation with advancing age and results because of an initial cytokine storm due to liver injury [73], (b) telomere shortening and features of cellular senescence in hepatocytes and cholangiocytes in cirrhosis [74-76], (c) immunesenescence of the liver resident and infiltrating immune cells by viruses [77] (d) changes in gut microbiota which in turn can influence the senescence of stellate cell in progression towards liver cancer [78]. Chronic liver disease with various etiologies showing different markers of cellular senescence has been tabulated (Table. 1). The progression from the initial insult to fibrosis, cirrhosis, and ultimately cancer is typically a prolonged process, highlighting the remarkable resilience and fail-safe mechanisms of liver cells in enduring such challenges over an extended period. Nevertheless, persistent chronic insults can disrupt these mechanisms, leading to the build-up of prematurely aged or senescent hepatocytes.
Senescence associated markers in liver disease
| Liver diseases | Senescence marker | References |
|---|---|---|
| Alcoholic liver disease | p21, mcm2 upregulatedCyclin A, PH3 downregulated | [82] |
| Non-alcohol-related fatty liver disease (NAFLD) | p21 upregulated, γ-H2AX, shortened telomere | [77] |
| nuclear vacuolation and increased nuclear area, p21, γ-H2AX expression, absence of Mcm-2 | [83] | |
| Chronic liver diseases | p21, p16 co-expressing with cyclin D1, NCAM | [84] |
| Primary biliary cirrhosis (PBC) | LC3 and p62 significantly correlated with the expression of p16, p21 | [85] |
| LC3 co-localizes with p16 and p21 | [86] | |
| Hepatocellular carcinoma | Cyclin D1, p21, γ-H2AX, IL6 | [87] |
| End Stage Liver Disease (ESLD) | p16INK4a, p21cip1 and p53 | [88] |
| p21 | [89] | |
| Acute liver failure | senescence marker protein (SMP30) | [90] |
| Liver fibrosis | p21, p53 and HMGA1 nuclear staining | [91] |
| NASH (Non-alcoholic steatohepatitis) | Increased beta-galactosidase activity and expressionIncreased p21WAF1, p16INK4A, p15INK4B, p53 and RB expressionDecreased expression of Ki-67, cyclin A and CDK2Formation of SAHF and SDF (e.g., HP1β, γ-H2A.X)Formation of DNA-SCARS (reflecting DDR or telomere dysfunction) | [92] |
| Human colon adenomas | IL-6, IL-8 | [93] |
| Acute rejection | p21WAF1/Cip, γ-H2A.X | [94] |
| Biliary atresia, primary sclerosing cholangitis, cellular rejection, and primary biliary cirrhosis | p15INK4B, p16INK4a, p21WAF1/Cip1, p53, Senescence Associated β-galactosidase | [95] |
| Biliary Atresia | Telomere length | [96] |
| Chronic hepatitis B infectionChronic viral hepatitis | Cyclin D1, p21, nuclear area | [97] |
| Senescence Associated β-galactosidase, p21 | [98] | |
| Chronic liver disease | p21, p53, and p16, HMG1-C, HP1γ, IL8 | [99] |
| Cryptogenic liver disease | p21, p27, p53 and Lamin B1 | [67] |
By employing retrospective radiocarbon (14C) birth dating of using hepatocyte nuclei from non-diseased human liver tissues (autopsy samples), a recent study revealed persistent renewal of human hepatocytes throughout a person's life with an average age of hepatocyte consistently below three years[79]. Based on genomic14C concentrations, the authors report that hepatocytes undergo renewal with a turnover of 19% per year and maintaining an average age of 2.7 years in younger individuals (25 years). Notably, this turnover rate is 17% per year in old age group (75 years) with an average hepatocyte age of 2.9 years. Additionally, the authors observed that polyploid cells have reduced turnover rates and probably reflective of less erosion of telomere thereby a protective mechanism against replicative senescence. In summary, this intriguing study demonstrates that hepatocytes possess inherent mechanisms that confer resilience to the aging process of the liver. Further recent study has indicated blocking ferroptosis shifts the liver transcriptome in older mice to resemble that of younger mice [80].
5ConclusionsThe idea that the liver "does not age" is due to its ability to maintain its function over time, unlike other organs, and it appears to age more slowly due to its exceptional regenerative ability. This regenerative capacity helps maintain liver function throughout albeit with certain age-related changes. This unique feature of the liver is due to the adaptive nature of its specialized cells, viz., hepatocytes. The adaptive mechanisms of increasing cell size, sustained telomere length, “ploidy conveyor,” cellular plasticity and organelle adapted stress response pathways provides hepatocytes with advantage to safeguard its function with chronological ageing. The fact that hepatocytes can be reprogrammed to younger state by chromatin reprogramming opens new avenues in setting of organ transplantation. Indeed, hepatocytes exhibit remarkable resilience to stressors, a quality that aligns with the “Red Queen hypothesis.” This is because the liver is an active metabolic organ and hence endowed with the adaptive capability to withstand challenging conditions of ever-changing metabolic conditions and environment within the body.
Scientific debate will remain on whether liver ages or not; nonetheless, it has been immortalized in verses of poetry and mythology. The ancient Greek mythology of Prometheus with immortal liver is well known and cited often. However, a lesser-known fact about liver is its metaphoric use immortalizing liver as an organ of passion and compassion in Urdu and Persian mystical poetry. The symbolic use of the word Jigar (meaning Liver) in mystical poetry has immortalized liver throughout ages in the Indian subcontinent. In Urdu poetry liver is often referred as “Jigar” which in turn has been romanticized as the powerhouse of “undying immortal” passion. The famous Persian mystic poet, Rumi, had metaphorically described the various organs and signified the strength and importance of liver in comparison to heart. “The body is a mine of endurance, the heart is a mine of gratitude, the bosom is a mine of laughter, the liver is a mine of compassion” (Mystical poems of Rumi). And ancient text of Sanskrit has immortalized liver through its verse “ yakrit sharisya pratibimbah” i.e. Liver is the mirror image of your body. The famous Chilean poet and Nobel Laureate, Pablo Neruda in his “ode to liver” writes about young liver “ as a maiden in the river laughs” and when the liver ages “ maiden is silent in the river” [81].